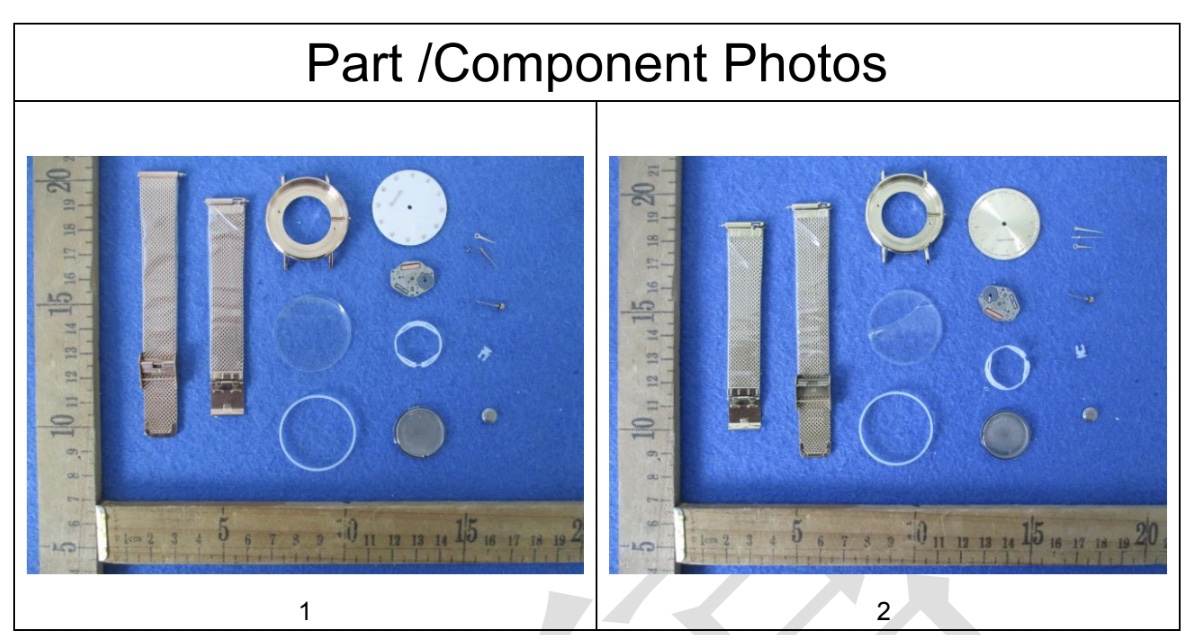
Background
Drevedy Watches is a brand owned by Love o Clock AB, based in Sweden. The company developed a new watch collection for launch in the autumn of 2022. This case study details how we helped them achieve the following through the Compliance Gate Platform:
1. Research relevant EU compliance requirements
2. Create mandatory documentation
3. Create label files
4. Book lab testing
Video
Content Overview
Step 1: Create requirements list
The first step of any process is to research relevant requirements. This is how we did it:
1.1 Add new product
1.2 Create new list
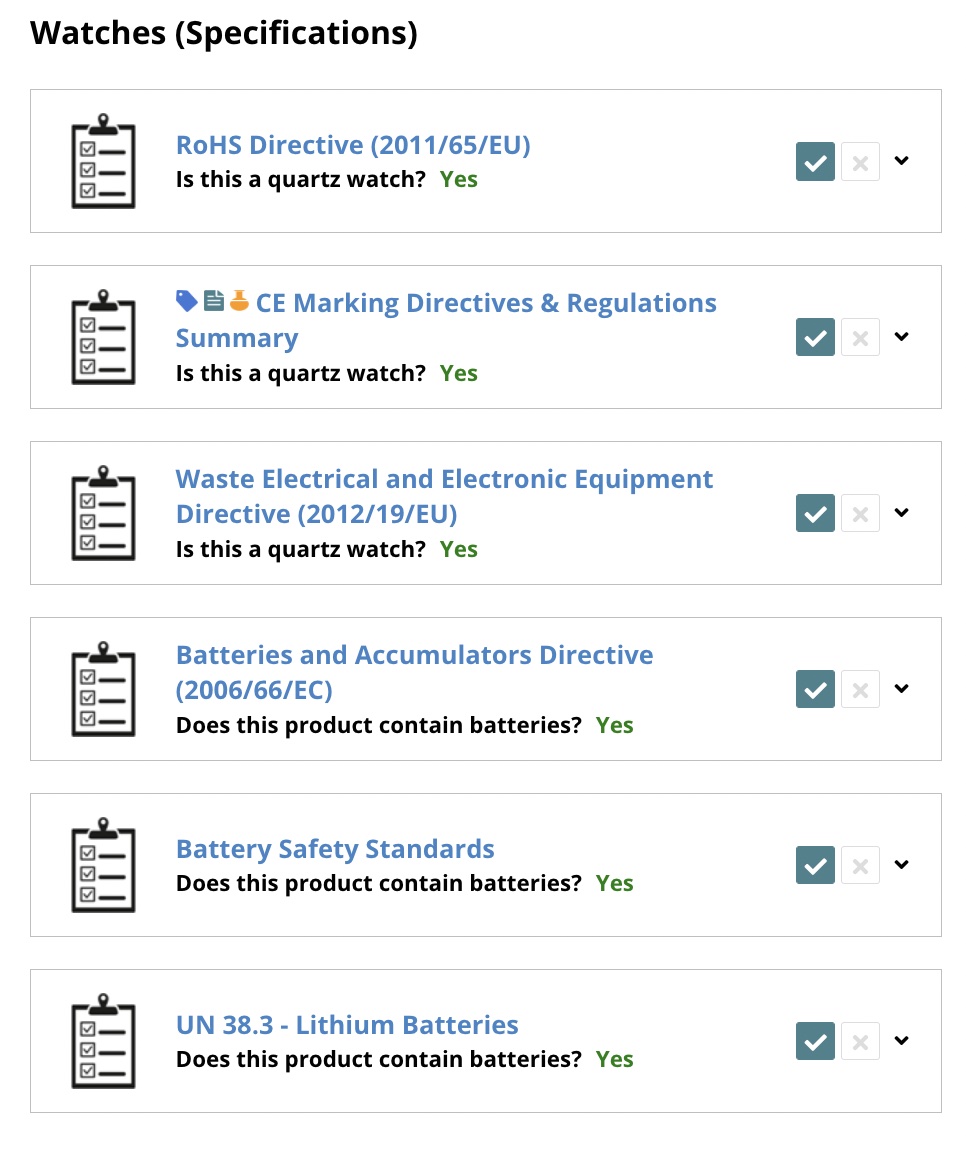
1.3 Review requirements
We now had a requirements list which can serve as a first step towards ensuring compliance in practice.This case study details how we helped them achieve the following through the Compliance Gate Platform:
Result
Here are some important highlights from the requirements list:
- Lab testing is required (RoHS, REACH, Battery Directive)
- We need to create an RoHS Declaration of Conformity
- We need to create RoHS label files
Step 2: Book lab testing
The requirements list informed us that lab testing is in practice mandatory. Hence, we helped the company arrange the following:
- RoHS testing
- REACH Annex XVII testing
- REACH SVHC testing
- Battery directive testing
- California Proposition 65
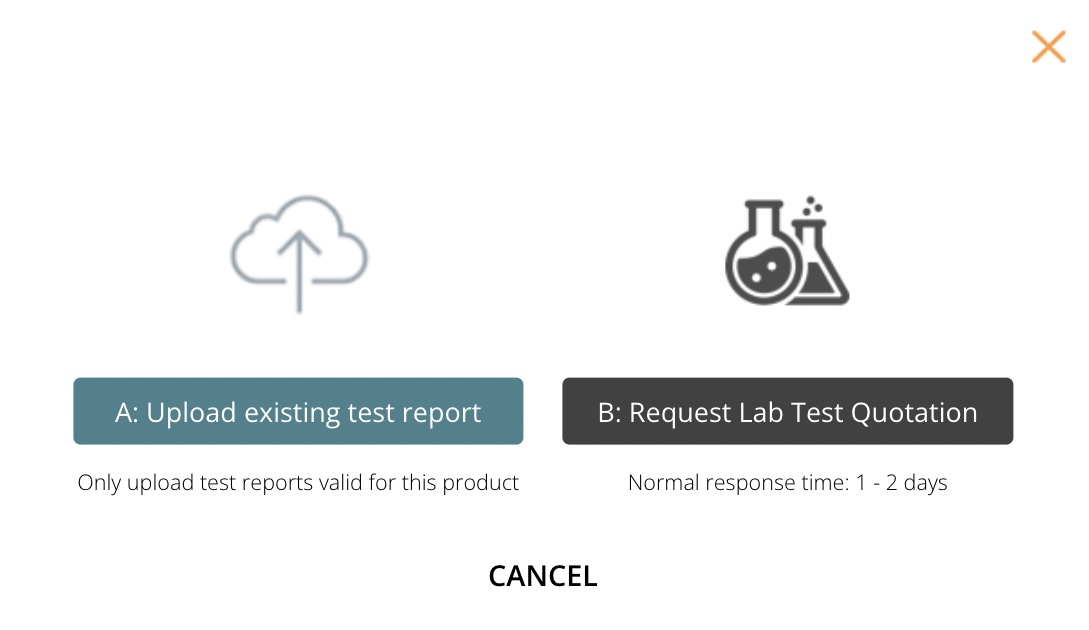
2.1 Request lab test quote
The first step of the process is to request a quotation. We helped the customer submit the quote request using the form.
Within 2 days, we received a first test quotation from QIMA, with a second test quote following a few days later from Testxchange.
Drevedy Watches ended up choosing the latter. The supplier then submitted samples to the lab facility, located in China.
2.2 Test result
Drevedy Watches received the test report in mid August 2022. The watches had passed the tests.
Step 3: Create RoHS Declaration of Conformity (DoC)
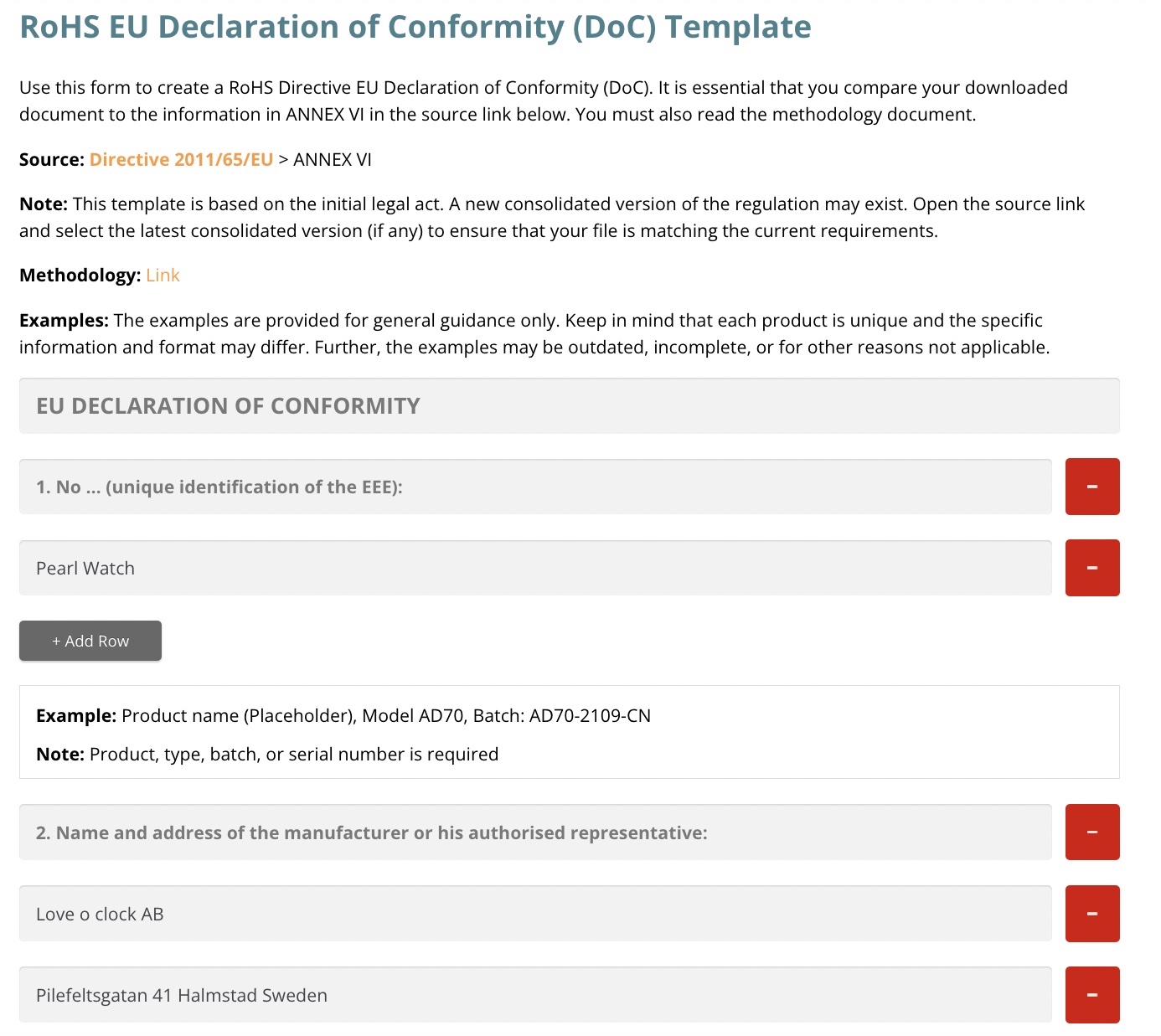
Now that the test result was in we could move forward and create one Declaration of Conformity. We used the template to create one DoC for each watch model.
Creating a DoC is mandatory for all products that must be CE marked, including quartz watches. This document can be requested by the customs or market surveillance authorities.
Failing to present a DoC can result in a recall, and fines.
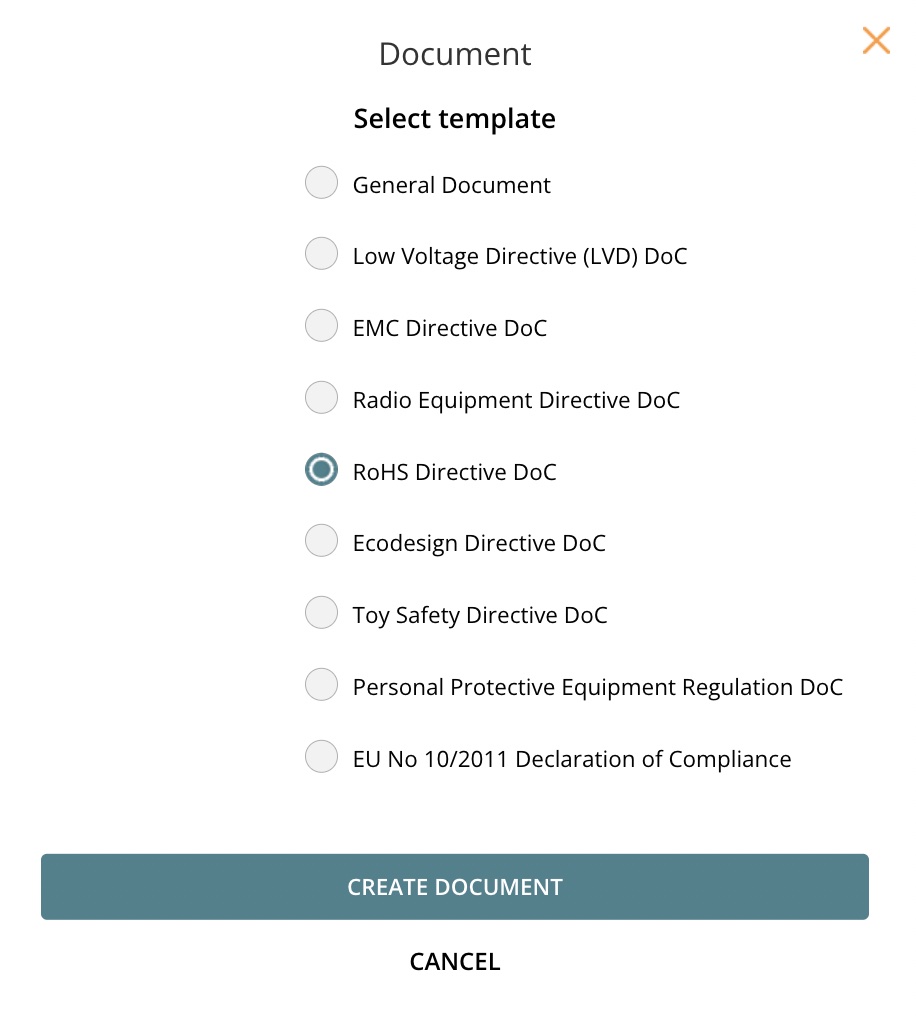
3.1 Select template
3.2 Create DoC
3.3 Downoad DoC
You can download a PDF copy of the Declaration of Conformity here.
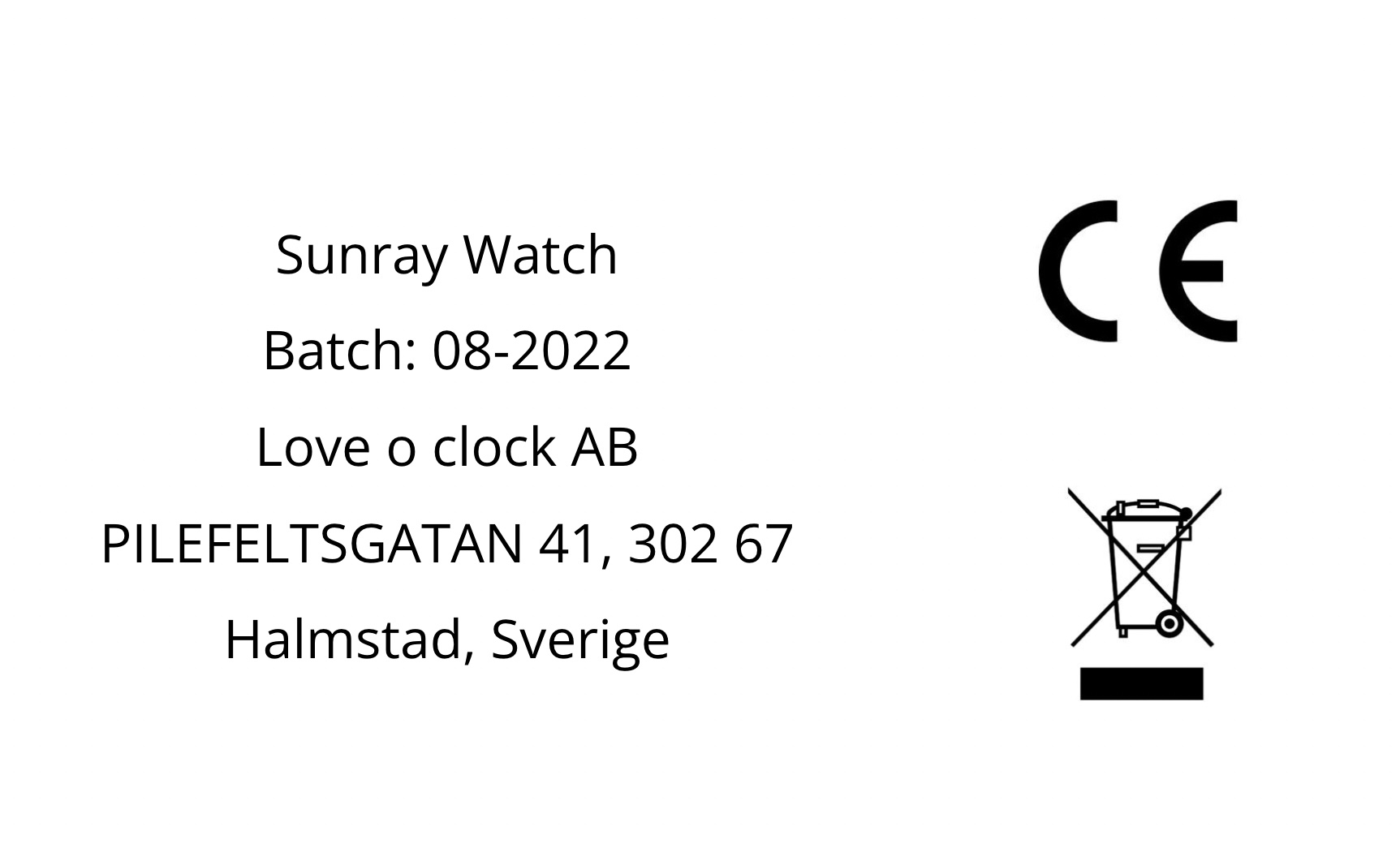
Step 4: Create RoHS label files
Now that we had created the DoCs it was time to create RoHS label files. We used the Compliance Gate Platform templates.
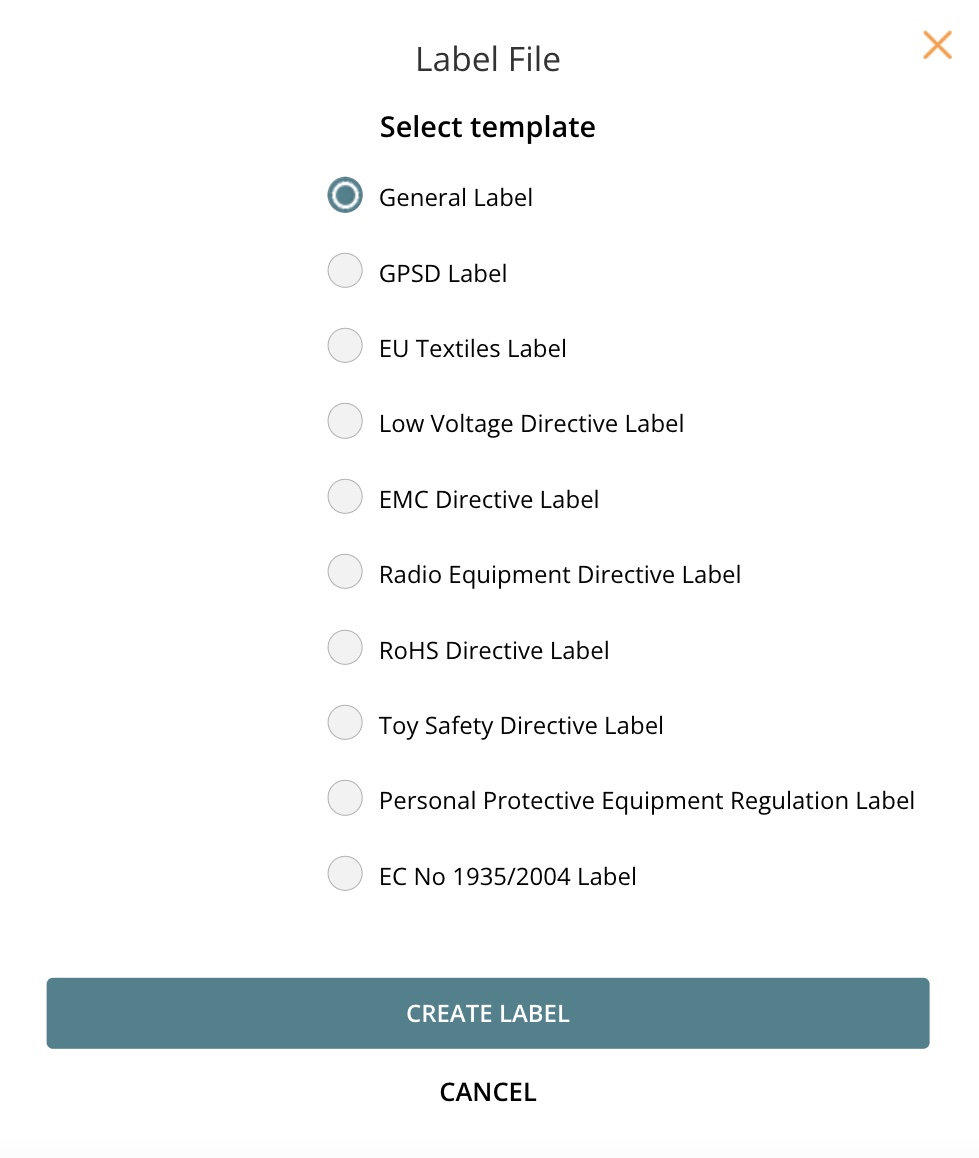
4.1 Select template
4.2 Create file
4.3 Download label
Note that we had to add the CE mark and WEEE symbol manually.
Step 5: Technical documentation
Finally, we had to help Drevedy Watches create a technical file. The Compliance Gate Platform does not come with a template for this purpose. However, the requirements list helped us identify a useful source on the EU website.
Some of these items were already available, such as the DoC, label files, and test reports. We then create a single file that also included the Bill of Materials and other requested information.