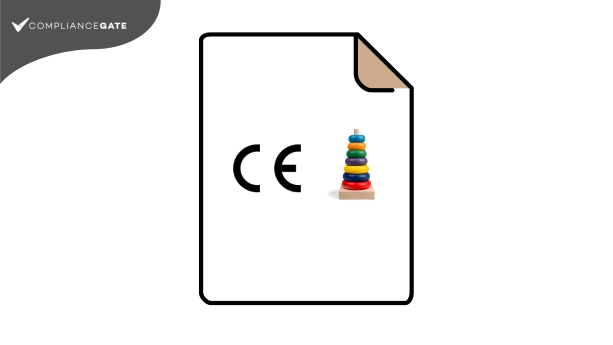
This guide explains how to create an EU Declaration of Conformity in accordance with Annex III of the Toy Safety Directive. We explain what to include under each of the 7 points of the document, and address common questions.
Continue reading How to Create an EU Declaration of Conformity for Toys















 Create compliance checklists for your product (US, EU & UK)
Create compliance checklists for your product (US, EU & UK) 20+ product certificate templates
20+ product certificate templates Create label files
Create label files Book product testing
Book product testing