
Are you importing or manufacturing food contact materials (FCM) products for the UK market? Applicable regulations might vary according to the type of product and material. Also, depending on the product, you would have to observe FCM regulations which may continue to come from EU regulatory sources.
This article covers documentation, labelling and other requirements that are applicable to various FCMs. This article does not cover FCM regulations for Northern Ireland.
Content Overview

FREE CONSULTATION CALL (US, EU & UK)
- Request a free 30-minute call with Ivan Malloci to learn how we can help you with:
- Find product requirements
- Certification and labeling
- Lab testing
What are Food Contact Materials?
FCMs are those that are intended to come into contact with food or are reasonably expected to be brought into contact with food. Here are some materials that are typically found in such products:
- Plastics
- Rubber
- Paper
- Metals
Materials and Articles in Contact with Food National 2012 Regulations
Pre-Brexit, the Materials and Articles in Contact with Food (England) Regulations 2012 (National Regulations) were in line with EU FCM legislation rules. Although the legislation has “England” attached to its title, the National Regulations apply to the United Kingdom.
Currently, the UK continues to keep up with EU FCM-related rules by virtue of the Materials and Articles in Contact with Food (Amendment) (EU Exit) Regulations 2019.
Note that importers and manufacturers should be citing UK legislation (as opposed to EU legislation) when going through necessary legal procedures – such as filling out the Declaration of Compliance.
Additionally, the National Regulations are worth observing as it contains information concerning non-compliance.
Finally, as a result of the UK’s departure from the EU, importers and manufacturers should be vigilant to changes that may occur in the legislation.
Product scope
The National Regulations 2012 and its retained EU regulations cover most FCMs including:
- Lunchboxes
- Electronic kitchen appliances
- Drinkware
- Stainless steel bottles
- Cutlery
- Bowls
- Jugs
- Tableware
Retained EU regulations: (Amendment) (EU Exit) Regulations 2019
The Materials and Articles in Contact with Food (Amendment) (EU Exit) Regulations 2019 amended the National Regulations 2012 and retained the EU FCM legislation. This ensures that there is continuity past the withdrawal date whilst ensuring that the responsibilities previously held by EU regulators are taken up by UK regulators.
This means that the following EU regulations should be observed when considering importing or manufacturing FCM products:
a. Regulation 1935/2004 (EU FCM Framework Regulation)
b. Regulation 1895/2005 (Epoxy derivatives used on FCM)
c. Regulation 2023/2006 (Good manufacturing practices for FCMs)
d. Regulation 282/2008 (Recycled plastic materials)
e. Regulation 450/2009 (Active and intelligent materials)
f. Regulation 10/2011 (Plastic materials and articles)
g. Regulation 2018/213 (On the use of bisphenol A in varnishes and coatings intended to come into contact with food)
h. Directive 84/500EEC (Ceramic articles)
i. Directive 2007/42/EC (Regenerated cellulose film)
Ceramics – Materials and Articles in Contact with Food Regulations 2012
Although the National Regulations reference EU Directive 84/500/EEC, they also provide specific provisions for ceramic articles.
Specifically, they restrict the migration limits of lead and cadmium. They also reference testing methods for evaluating the migration limits.
Substance restrictions
Article 10, Part 4 of the legislation contains the limits for lead and cadmium from ceramic articles.
Declaration of Compliance
Ceramic articles which are not yet in contact with foodstuffs should be accompanied by a written declaration as per the FCM Framework regulations. Schedule 4 contains the content required in the Declaration of Compliance (DoC).
Appropriate documentation demonstrating compliance should be made available.
Food Contact Materials Framework Regulation ((EC) 1935/2004)
The Food Contact Materials Framework Regulation continues to be applicable in the UK context. Its purpose is to regulate FCMs, including active and intelligent FCMs and articles, in the UK market to ensure that such products are safe to end users. To this end, the legislation imposes both general and specific requirements for FCMs that importers and manufacturers must observe.
Scope
The scope of this legislation includes:
a. Materials and articles that in their finished states are in contact with or are expected to be in contact with food
b. Active FCMs and articles (these are intended to extend the shelf-life or to maintain or improve the condition of packaged food)
c. Intelligent FCMs and articles (these are tools which monitor the condition of packaged food or the environment surrounding the food)
The legislation states that specific measures may apply to certain groups of materials and articles (in addition to the requirements contained in this legislation), like:
- Active and intelligent materials and articles
- Ceramics
- Rubbers
- Glass
- Metals and alloys
- Paper and board
- Plastics
- Regenerated cellulose
- Textiles
- Wood
General requirements
Generally, FCMs should comply with the good manufacturing practice when manufacturing FCMs to ensure that they do not transfer their constituents to food which could be either:
a. Dangerous to human health
b. Result in an unacceptable change in the composition of the food article
c. Result in the deterioration of the organoleptic characteristics (such as flavour, texture and colour) of the food article
Additionally, it is a general requirement that FCMs are labelled, advertised and presented in a manner that does not mislead consumers.
Special requirements
Special requirements exist for active and intelligent FCMs and articles. Here are some examples of the special requirements:
a. Active FCMs and articles must not bring about changes that would mask the spoilage of food, misleading consumers
b. Such materials and articles should be adequately labelled to indicate that they are active and/or intelligent
Declaration of Compliance (DoC)
The legislation stipulates that the specific measures covering specific groups of materials and articles require such items to be accompanied by a written declaration. The written declaration should state that the materials and articles comply with the rules applicable to them, and that appropriate documentation should be made available to show compliance.
Labelling requirements
Generally, importers and manufacturers placing in the UK market FCMs, which are not yet in contact with food, should abide by the following labelling requirements:
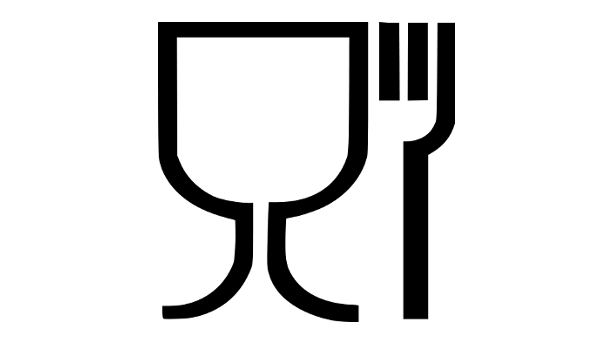
a. Display the words ‘for food contact’ or a specific indication as to their use such as the wine glass and fork symbol found in Annex II (when it is not obvious that it would be in contact with food)
b. Provide special instructions for safe and appropriate use (if necessary)
c. Provide the name or trade name and the address of the importer or manufacturer responsible for placing the FCM product in the market
d. Provide adequate labelling or identification to ensure traceability
e. Provide information on the permitted use or uses and other relevant information (such as the name and quantity of the substances released) of active FCMs and articles to enable food business operators using such materials and articles to comply with relevant regulations (such as food labelling regulations)
Generally, the above labelling information should be conspicuous, clearly legible and indelible.
Lab testing
The legislation states the specific measures may include a list of authorised substances for use in manufacturing, purity standards for such substances, migration limits, and so on for certain groups of materials and articles named in Annex I. Thus, lab testing would be necessary to ascertain such information and to ensure compliance.
Good Manufacturing Practice for FCM – Regulation ((EC) No 2023/2006)
Importers and manufacturers are required to ensure that manufacturing operations are carried out following the general rules and detailed rules (where applicable) on Good Manufacturing Practice (GMP) as set out in the legislation.
The general rules compel business operators to establish all of the following:
a. A quality assurance system
b. A quality control system
c. Establish and maintain appropriate documentation which is relevant to compliance and safety of the finished material or article
Detailed rules on GMP are available for:
a. Printing inks (the rules concern the processes involving the application of printing inks on the non-food contact side of a material or article)
b. Quality assurance systems for plastic recycling processes covered by Regulation (EC) No 282/2008 on recycled plastic materials and articles intended to come into contact with foods
Plastic Materials – Regulation (EU) No 10/2011
This legislation contains special measures for plastic FCMs. It regulates plastic FCMs that fall under the following categories:
a. Materials and articles and parts consisting of plastics
b. Plastic multi-layer materials and articles held together by adhesives or by other means
c. Materials and articles referred to in points a. or b. that are printed and/or covered by a coating
d. Plastic layers or plastic coatings, forming gaskets in caps and closures, that together with those caps and closures compose a set of two or more layers of different types of materials
e. Plastic layers in multi-material multi-layer materials and articles
Authorised substances
The regulation lists authorized substances, which are the only substances that can be used during the manufacturing of plastic FCMs. The list can be found in Annex I of the legislation. Substances that are not listed as authorised substances cannot be used, unless by way of derogation.
Substance limits
The regulation sets general and specific substance restrictions (e.g. limits on the amount of certain substances in the material) and migration limits. These can be found in chapter 2, section 2 of the legislation.
Declaration of Compliance
Information that should be contained in the DoC is located in Annex IV. It should allow for the easy identification of the items or substances for which it is issued, and should be renewed when substantial changes are made that affect the migration from the materials or articles or when new scientific data is available.
Appropriate documentation demonstrating compliance with this legislation should be prepared by the business operator. Typically, lab reports would be included in such documentation.
Active and Intelligent Materials – Regulation (EC) No 450/2009
This is a retained EU legislation that regulates the use of active and intelligent materials and articles (AIMs) placed in the UK market.
Authorized substances
The legislation states that only authorized substances may be used in components of AIMs unless exempted by way of derogation. Authorised products would be listed in a ‘community list’ according to the legislation.
Such lists containing authorised items are also referred to as positive lists. However, It should be noted that positive lists for AIMs have yet to be established. Until such a list is established, AIMs may be placed if they meet:
- The requirements of the General Food Law Regulations
- Any general criteria in the FCM legislation
Additional considerations may apply (such as if the AIMs contain a biocidal substance, they may need to be assessed by the Health & Safety Executive (HSE) for suitability as an FCM).
Declaration of Compliance
The DoC should contain the information contained in Annex II. Appropriate documentation to demonstrate compliance with the requirements of this regulation should be made available by the business operator. Typically lab reports would be included in such documentation.
Labelling requirements
There are additional rules on labelling for AIMs where non-edible parts may be perceived as edible by consumers. If such a risk is present, a label containing the following should be displayed:
a. The words ‘DO NOT EAT’
b. The symbol presented in Annex I (where possible)
Recycled Plastic Materials – Regulation (EC) No 282/2008
This regulation sets additional requirements for recycled plastic materials and articles intended to come into contact with foods, including the following:
a. Only recycled plastic obtained from a single recycling process can be used
b. The recycling process should be managed by an adequate quality assurance system
Declaration of Compliance
Additional requirements are there for the DoC relating to recycled plastic materials and recycled plastics. Part A and Part B of Annex I to the legislation respectively should be observed.
Labelling requirements
Voluntary self-declaration of the recycled content in recycled plastic materials and articles should follow the rules contained in ISO 14021:1999 or an equivalent version.
Regenerated Cellulose Film – Directive 2007/42/EC
This directive is retained. It sets additional requirements for regenerated cellulose film (RCFs) intended to come into contact with food.
Authorized substances
The legislation lists substances that are authorized to be used in these kinds of products. The list is divided into two parts:
- Uncoated regenerated cellulose film
- Coated regenerated cellulose film
For each part and each substance, the directive might also provide a restriction limit.
The list of authorised substances in the manufacture of RCF is contained in the first and second part of Annex II.
Declaration of Compliance
The DoC requirements contained in the FCM Framework regulations should be followed for materials and articles made of RCFs unless such RCFs are by their nature clearly intended to come into contact with foodstuffs.
Labelling requirements
Materials or articles made of RCFs shall be labelled accordingly where special conditions of use are indicated.
The N-nitrosamines and N-nitrosatable Substances in Elastomer or Rubber Teats and Dummies (Safety) Regulations 1995
This directive sets additional requirements when importing teats and soothers containing elastomer or rubber. In particular, it sets the following substances migration limits:
a. N-nitrosamines < 0.01 mg/kg of the parts of teat or soother made of elastomer or rubber
b. N-nitrosatable substances < 0.1 mg/kg of the parts of teat or soother made of elastomer or rubber
FCM from China or Hong Kong SAR (China) – Regulation ((EU) No 284/2011)
The Food and Feed Imports (Amendment) (EU Exit) Regulations 2019 retains Commission Regulation (EU) No. 284/2011.
This regulation requires that plastic kitchenware/tableware originating from or consigned in China and Hong Kong SAR (China) should be imported into the UK unless the importer provides a declaration and a laboratory report confirming that the related products are compliant with limits on the release of harmful chemicals such as formaldehyde or primary aromatic amines.
Substance limits
A test report should be attached to the declaration, concerning the following substances detection limits:
a. Primary aromatic amines in polyamide kitchenware < 0.01 mg per kg of food
b. Formaldehyde in melamine kitchenware < 15 mg per kg of food
Epoxy Derivatives – Regulation (EC) No. 1895/2005
This regulation is retained and sets additional requirements for the following FCMs:
- Plastic products or products that contain plastic components
- Products covered by surface coating
- Adhesives
Substance limits
The regulation sets migrations limits or bans in use of certain epoxy derivatives, including:
- BADGE and some of its derivatives
- BFDGE
- NOGE
Declaration of Compliance
A DoC drafted as per the requirements in the FCM Framework regulations should be made available, and appropriate documentation should be provided to demonstrate compliance.
Infant Cups and Bottles – Use of Bisphenol A in Varnishes and Coatings Regulation ((EU) 2018/213)
This retained regulation bans the use of Bisphenol A in materials used to manufacture:
a. Polycarbonate infant feeding bottles
b. Polycarbonate spill-proof drinking cups or bottles that are intended for infants and young children
Declaration of Compliance
As per the FCM Framework regulations, business operators should ensure that varnished or coated materials and articles are accompanied by a DoC containing the information laid down in Annex I to the regulation.
Application for authorisation of novel FCM
The Food Standards Agency (FSA) and the Food Standards Scotland (FSS) are responsible for ensuring that products relating to food in the UK are safe, and are responsible for food hygiene.
Amongst the various regulated products, the agencies regulate FCMs which may require authorisation and may prevent the importer or manufacturer from placing the FCM in the UK.
Novel FCM
FCMs that have been authorised are listed in the relevant legislation (also known as the positive list), and the legislation will set out how they can be used and associated conditions of use (if any).
However, there may be FCMs that aren’t listed in the positive list. These “novel” FCM substances need to be submitted for evaluation and consideration for their ultimate inclusion in the positive list.
The retained EU legislations set out authorisation requirements for the following FCMs:
- Plastic monomers and additives
- AIMs
- Recycled plastic processes
- Regenerated cellulose film
National Reference Laboratories (NRL)
NRLs are specialist laboratories responsible for maintaining standards for the routine testing of feed, food and animal health. For FCM products, FERA is the relevant NRL.
When seeking authorisation, a product sample must be submitted to NRL (in this case FERA) for the following FCMs:
- Additives and starting monomers in plastic FCMs
- Additives in active and intelligent FCMs
- Additives in regenerated cellulose film
Authorisation procedure
For novel FCMs which are not listed under any current legislation, you would have to:
a. Use the application service
b. Upload your application documents which will form your dossier
c. Send a physical sample and accompanying information to FERA
You should submit the following to FERA’s address:
a. A physical sample of the substance (250g)
b. The relevant product safety sheet and spectroscopic data (when applicable)
c. The analytical method(s) including performance parameters which are found in items 5.1.8, 5.3,7 and 6.5 of Note for Guidance for Food Contact Materials
d. Your contact details
The Environmental Protection (Single-use Plastic Products) (Scotland) Regulations
Similar to the EU Single-use Plastic Directive, Scotland has introduced legislation banning the supply and manufacturing of single-use expanded polystyrene and plastic products in the jurisdiction. The legislation took effect on 1st June 2022.
The aim of the ban is to encourage businesses to turn to reusable alternatives resulting in reduced litter and emissions. Committing an offence under the legislation may result in a maximum fine of GBP 5,000.
The legislation only applies to products manufactured in or imported directly into Scotland. However, from 12 August 2022, it will be unlawful to manufacture and supply the products listed below, regardless of whether they are produced or first imported into another part of the UK.
Product scope
The legislation covers the following single-use expanded polystyrene and plastic products:
a. Expanded polystyrene beverage containers (this includes any cover or lid)
b. Expanded polystyrene beverage cups
c. Expanded polystyrene food containers
d. Plastic beverage stirrers
e. Plastic cutlery designed and intended for eating or serving food (this includes knives, forks, chopsticks, spoons and other similar utensils)
f. Plastic plates
g. Plastic straws (subject to certain exemptions)
h. Plastic balloon sticks (however, supplying them for attaching to balloons for industrial or other professional uses and applications that are only distributed to persons acting in the course of a business is permitted)
Requirements
Business operators should not supply or manufacture the above products and commits an offence if they do so. However, there are exemptions for the supply of plastic straws:
a. Which are medical devices or are used for medical purposes
b. By retail pharmacy businesses
c. By catering establishments together with food or drink for immediate consumption
d. In certain establishments such as schools and care homes
e. For use in a support service which provides personal care or personal support
f. Which are packaging as defined in regulation 3 of the Packaging (Essential Requirements) Regulations 2015
Due diligence
A person charged under the legislation can defend against the charge by demonstrating that all reasonable precautions were taken and due diligence was exercised to prevent the offence from being committed.
Thus, you should check such product categories mentioned above if you are unsure whether they are made up of or contain plastics or expanded polystyrene.
General Product Safety Regulations
The General Product Safety Regulations 2005 (GPSR) ensures the safety of consumer products placed in the UK by imposing the general duty for producers to supply safe products.
Producer obligations include ensuring product traceability and adopting measures to enable relevant stakeholders to be informed of risks that the product might present.
In support of the GPSR, there are published standards that are voluntary in nature, but if followed will give rise to a “presumption of conformity”. If such standards do not cover your particular FCM product, then it will be assessed for safety by taking into account:
- Voluntary European standards or standards drawn up in the UK
- Industry codes of good practice
- The state of the art and technology
- Reasonable consumer expectations concerning safety
Product scope
The legislation applies to most consumer products, including FCM products, unless the product is affected by specific UK legislative provisions. Thus, they function as a “catch-all” set of regulations for such unaffected products.
Requirements
Importers and manufacturers should:
a. Provide consumers with relevant product safety information
b. Allow for traceability (including adding a traceability label to the product)
c. Ensure that the product is safe
d. Adopt a risk assessment system
e. Notifying distributors of actual or potential risks, and reporting to enforcement authorities actual risks to consumers. In the latter case, taking risk preventive measures
Referenced standards
Complying with a voluntary standard provides a presumption of conformity. Currently, one FCM-related standard is referenced:
a. BS-EN-14350-1 – Child use and care articles – Drinking equipment – Part 1: General and mechanical requirements and tests
Note: Contains public sector information licensed under the Open Government Licence v3.0.













 Create compliance checklists for your product (US, EU & UK)
Create compliance checklists for your product (US, EU & UK) 20+ product certificate templates
20+ product certificate templates Create label files
Create label files Book product testing
Book product testing