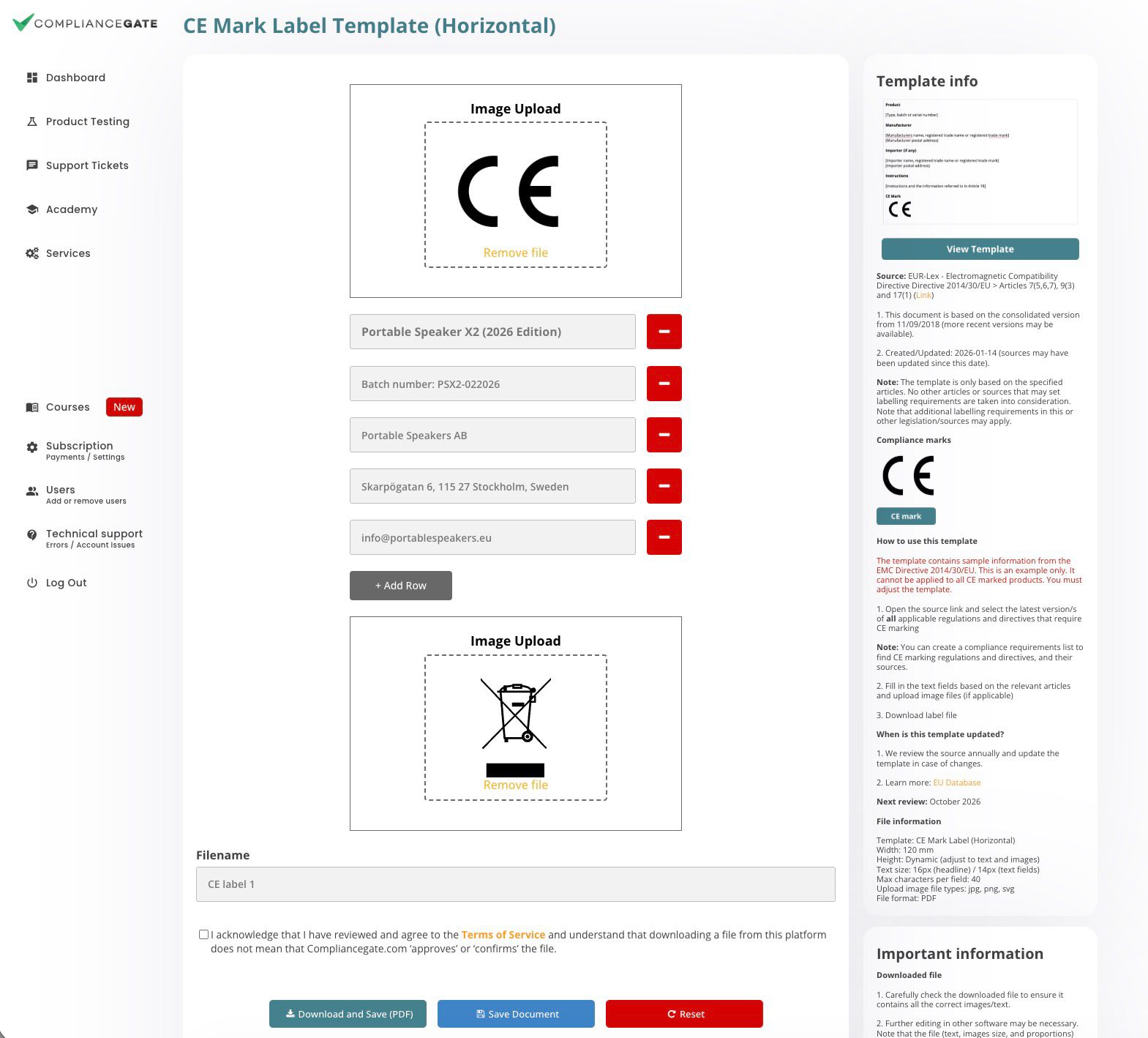
Label Templates
Why do I need to create label files?
Essentially all consumer products are subject to mandatory product and packaging labelling requirements. Our platform makes it possible to find requirements and create label files.
You can use a blank layout or a label template
Add label text and upload images (i.e., compliance marks or pictograms)
Step 3: Download label file (PDF)
The label file can be downloaded as a PDF and further edited in Canva or Photoshop
What should I do with the downloaded label file?
1. Labels can be affixed to the product, packaging, or accompanying documentation.
2. You can make further edits in Photoshop, Canva, or other software. You can also incorporate the label files into other files (i.e., packaging artwork).
3. You must ultimately follow the requirements as specified in the latest version of the relevant legislation, guidance pages or other sources.
Note: We recommend that you book a third-party label review before affixing label information on the product, packaging, or documentation.
.png)
US Label Templates

CPSIA Tracking Label – Standard
This template is based on CPSC guidance pages related to the CPSIA

CPSIA Tracking Label – Durable Infant Products
This template is based on CPSC guidance pages related to the CPSIA
.png)
EU Label Templates

General Product Safety Regulation
This label template is based on specific articles in the GPSR

CE Marking
This label template is based on specific articles in the EMC Directive
.png)
UK Label Templates

UK General Product Safety Regulation 2005
This label template is based on specific articles in the UK GPSR 2005

UKCA Marking
This label template is based on specific articles in the EMC Regulations 2016
Other Templates
Standard label layout
Need to create a document for which we don’t have a template? Use the blank document template.
You can find a list of limitations and risks related to the label templates in this document. 1. Certain legislation mandates the creation of declarations, certificates, technical documentation and other documents. Such documents can be requested by national authorities and marketplaces, such as Amazon. 2. The document templates are based on specified parts of legislation and/or official guidance documents. 1. Certain legislation sets requirements concerning marking and labelling requirements. Labels can, depending on the specific placement requirements, be printed on product, packaging, and/or accompanying documents. 2. The label templates are based on specified parts of legislation and/or official guidance documents. You can find a list of US, EU and UK templates (and the data and versions these are based on) in these documents: 1. We base document templates on what is written in legislation and/or guidance documents. 2. For example, the EU Declaration of Conformity document template is based on the EMC Directive 2014/30/EU > ANNEX IV. 3. Label templates are only based on the specified parts of a certain legislation. 1. Start by selecting a template relevant to your product. 2. You must carefully read the instructions in the right sidebar and follow these guidelines: Note: The templates are only updated during an annual audit in October. As such, the templates may be outdated if the source (legislation or guidance document) is updated after the annual audit. It is therefore important to check the latest source version. Note: You must ultimately create/affix/submit documents and labels based on the requirements in the latest version of the relevant legislation, guidance pages or other sources. Note that label placements can be conditional. Furthermore, the exact wording used in documents, instructions, warnings, and other files can be open-ended and not clearly specified in legislation or other sources. Note: Definitions in legislation may provide information about the particular economic operators and product details that may need to be present in documents and labels. 3. Add information to the fields and upload image files (when necessary). Note that some documents can only be issued if there is other supporting documentation, such as test reports. 4. Download a PDF copy of the document or label after completion and ensure that you maintain backups at all times. Document note: Certain regulations require that documents be maintained for a certain period of time (e.g., 10 years after you have placed a product on the market). Further, some documents must also be printed and signed. Label note: Further editing in other software may be necessary. We also recommend that you book a third-party label review before affixing label information on the product, packaging, or documentation. 1. Each document and label template is based on specified parts of legislation texts and/or official guidance pages/documents. 2. We primarily use data from the following sources: 3. Each document and label template entry is based on the source version available at the time of creation. 4. You can find information about the document template versions, sources, and how we manage updates in the following documents: You can find a list of limitations and risks related to the Document/Label Templates in this document. No, we only provide the templates listed here: We do not claim to provide templates for all documentation/certification/labelling requirements in existence in the mentioned markets, or that may apply to a particular product. The templates are only updated during an annual audit in October. As such, the templates may be outdated if the source (legislation or guidance document) is updated after the annual audit. It is therefore important to check the latest source version. The template must be adjusted based on the latest version of the relevant article, annex, regulations, part, or guidance document before it is filled in. Lacking the mandatory product certificates and other compliance documents can result in a recall, or that your products are removed from marketplaces such as Amazon. You can use the label templates as a starting point, but you must ultimately create label files based on the latest versions of all relevant regulations, guidance documents, and other sources. Note that a product can be subject to many regulations which all set labelling requirements.Feature Overview
What is a document template?
What is a label template?
Which templates are included?
How do you create document and label templates?
How should we use document/label templates?
Sources and versions
Limitations and risks
Do you provide templates for all documentation and labels required in the US, EU and UK?
How do you keep the document and label templates up to date?
What can happen if I don’t have the mandatory compliance documents?
How do we make sure that the label files are correct?