This guide explains how to create an EU Declaration of Conformity for PPE based on the model structure in Annex IX of the Personal Protective Equipment (PPE) Regulation. You will learn what to include under each of the 8 points, while I also address common questions.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
1. PPE (product, type, batch or serial number):
A Declaration of Conformity is generally only valid for a single product model, which must be clearly identified under point one. For a pair of sunglasses, it might look something like this:
Example
Sunglasses Style 07 (Black)
Batch: MMYYYY
2. Name and address of the manufacturer and, where applicable, his authorised representative:
Article 8(2) of the Personal Protective Equipment (PPE) Regulation states that the manufacturer is responsible for creating the Declaration of Conformity. That said, manufacturers are in this context not only covering actual factories, but also companies that order products from contract manufacturers or order branded products.
For example, a brand ordering sunglasses from a factory in China based on their design or with their brand would likely be deemed the manufacturer and therefore responsible for issuing the Declaration of Conformity.
If I use the sunglasses example again, this second part of the DoC could look like this:
Example
Dublin Eyewear Limited
720 S Circular Rd, Mountjoy, Dublin, D01 RP11, Ireland
Authorised representative
Non-EU manufacturers selling Personal Protective Equipment (PPE) directly to consumers in the EU must have an authorised representative. This company must also be specified on the DoC. However, this is only relevant in particular circumstances.
When doing so, the EU AR information is provided in addition to the manufacturer’s name and address.
3. This declaration of conformity is issued under the sole responsibility of the manufacturer:
This is a statement where the manufacturer declares their responsibility to ensure compliance under the Personal Protective Equipment (PPE) Regulation.
4. Object of the declaration (identification of PPE allowing traceability; where necessary for the identification of the PPE, a colour image of sufficient clarity may be included):
Under this point, you can include an image showing the product specified under point 1. Below follows an example for eyewear.

If the same product is available in multiple colours, then these can also be included here.
5. The object of the declaration described in point 4 is in conformity with the relevant Union harmonisation legislation: …
You must list all EU regulations and directives that the covered PPE product is in compliance with. In this specific case, this must at a minimum list the Personal Protective Equipment Regulation (EU) 2016/425.
That being said, there are situations in which products covered by the Personal Protective Equipment (PPE) Regulation are subject to more than one regulation or directive. In such cases, you must also list other applicable regulations and directives that also require a Declaration of Conformity.
Examples
1. PPE that contains electronic components
2. PPE with measuring instruments
3. PPE that includes toys or components with play value (as defined under the Toy Safety Directive)
6. References to the relevant harmonised standards
Full text: References to the relevant harmonised standards used, including the date of the standard, or references to the other technical specifications, including the date of the specification, in relation to which conformity is declared
You must list all harmonised standards which your product complies with. Below follow examples of harmonised standards under the Personal Protective Equipment (PPE) Regulation:
Sunglasses
EN ISO 12312-1 – Eye and face protection – Sunglasses and related eyewear – Part 1: Sunglasses for general use
EN ISO 12312-2 – Eye and face protection – Sunglasses and related eyewear – Part 2: Filters for direct observation of the sun
Helmets
EN 397 – Industrial safety helmets
EN 443 – Helmets for fire fighting in buildings and other structures
EN 966 – Helmets for airborne sports
EN 1078 – Helmets for pedal cyclists and for users of skateboards and roller skates
EN 1080 – Impact protection helmets for young children
EN 1384 – Helmets for equestrian activities
Work gloves
EN 12477 – Protective gloves for welders
EN 659 – Protective gloves for firefighters
EN 421 – Protective gloves against ionizing radiation and radioactive contamination
Test reports
Note that all PPE standards listed under this point must also be listed in your test reports. You should also ensure that the specific data and version, as written in the test report, is specified.
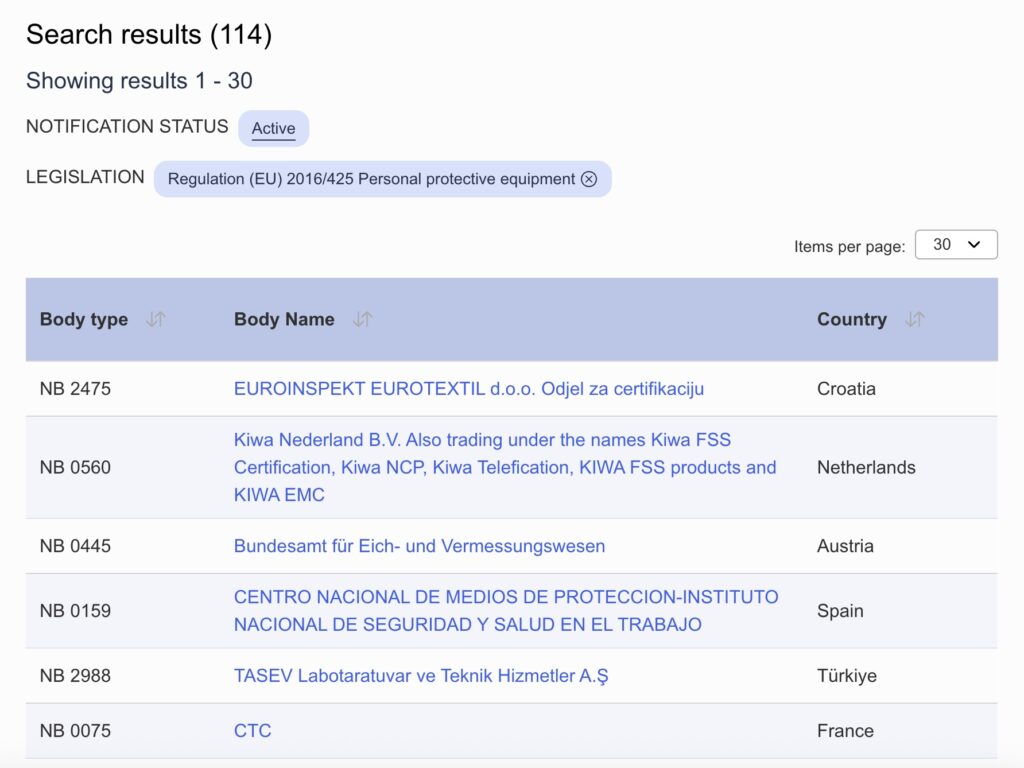
7. Notified body information (Module B)
Full text: Where applicable, the notified body … (name, number) … performed the EU type-examination (Module B) and issued the EU type-examination certificate … (reference to that certificate).
Personal Protective Equipment (PPE) classified as category II or category III requires the involvement of a notified body. As such, if your product belongs to either of these categories, which in turn depends on the risk, you must specify the following information about the notified body involved in the conformity assessment procedure:
- Company name
- Notified body number
- EU type-examination certificate number
The specific company name and number of the notified body should match what is registered in the EU NANDO database.
Example
Is this part required for PPE Category I?
No, PPE category I generally do not require the involvement of a notified body. As such, there is no notified information to specify in the Declaration of Conformity.
8. Notified body information (Module C2 or Module D)
Full text: Where applicable, the PPE is subject to the conformity assessment procedure … (either conformity to type based on internal production control plus supervised product checks at random intervals (Module C2) or conformity to type based on quality assurance of the production process (Module D)) … under surveillance of the notified body … (name, number).
Products defined as PPE category must, in addition to Module B listed under point 7, also undergo one of the following conformity assessment procedures:
- Module C2: Internal production control plus supervised product checks at random intervals
- Module D: Conformity to type based on quality assurance of the production process
The notified body that was involved with either Module C2 or Module D must be specified under point 8.
9. Additional information:
Finally, a person employed by the manufacturer signs the Declaration of Conformity according to the following format:
Signed for and on behalf of: …
(place and date of issue):
(name, function) (signature):
Note that the PPE Declaration of Conformity must be stored for at least 10 years after the product is no longer sold in the European Union.
FAQ
How do we specify the date and version of a standard?
Standards harmonised under the PPE regulation can be subject to amendments and thus updated, while older versions are phased out. In practice, this is done as PPE standards are improved based on industry input, data and other factors.
It must therefore be clear which PPE standard your product is in compliance with.
Do we need to include the DoC with the PPE product?
Yes, for PPE, it is a requirement to either include the EU Declaration of Conformity with the product or at least provide a website address in the PPE instructions where a copy can be found.
Does the Declaration of Conformity format differ for different PPE categories?
The model structure is the same for PPE categories I, II and III. That said, point 7 is only relevant for PPE category II and III, while point 8 is only relevant for PPE category III.
Is it mandatory to assign a number to the DoC?
No, the Personal Protective Equipment (PPE) Regulation states that this is optional. However, doing so can help you keep track of multiple DoCs, as you must generally create one per product model.



















.png)
.png)
.png)