Products can only be correctly CE marked if the manufacturer can provide a set of clearly defined documents. In this guide, we explain what these documents are and their relevance to the CE marking process.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
Declaration of Conformity (DoC)
A Declaration of Conformity (DoC) is a statement issued by the manufacturer, declaring that a certain product is in compliance with the specified regulations, directives, and (in most cases) European standards. The Declaration of Conformity (DoC) must also be dated and signed.
You can find a model structure in most, if not all, EU regulations and directives mandating CE marking. That said, the exact information differs. You may need to combine elements from multiple model structures when creating a Declaration of Conformity.
Further, the Declaration of Conformity must be saved for 10 years after you have discontinued a certain product.
It should also be noted that a Declaration of Conformity alone is of no use unless it is supported by test reports and technical documentation.
Declaration of Conformity Sample
Test Report
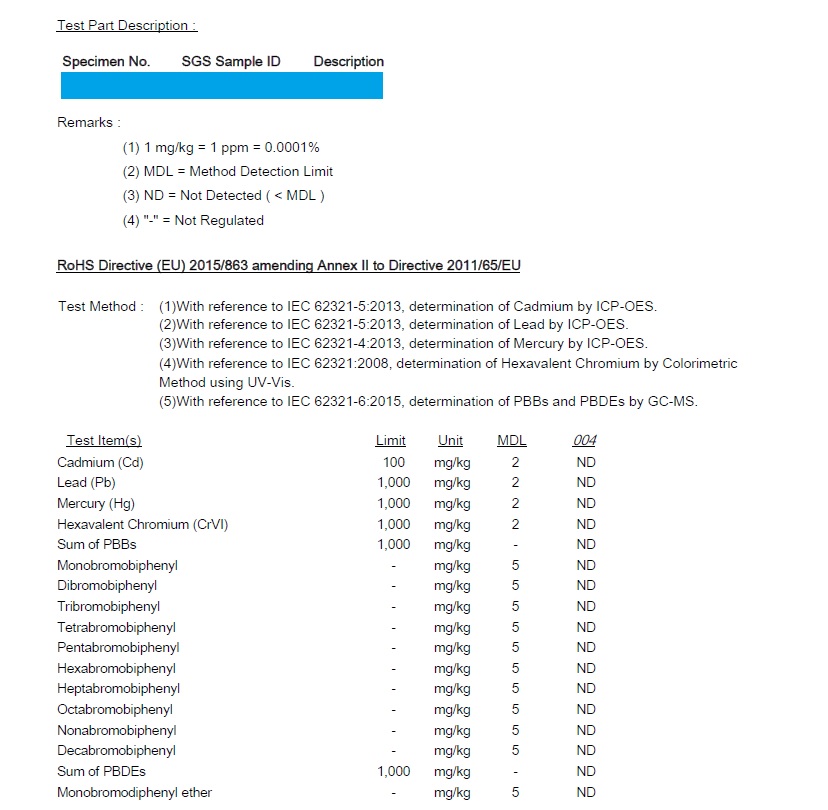
Test reports specify if a certain product has passed testing according to the listed standards. Assuming the product is compliant, the test report should indicate that the result is passed.
As such, a test report serves as evidence that a product is compliant with relevant European standards. This is essential for CE marked products, which must comply with harmonised or other product standards.
To obtain a test report, you must normally contact a third-party lab like TUV Rheinland or SGS. While third-party testing is not always mandatory for CE marked products, most businesses do not have the in-house equipment and expertise to carry out testing according to applicable product standards.
RoHS Test Report Samples
Instructions
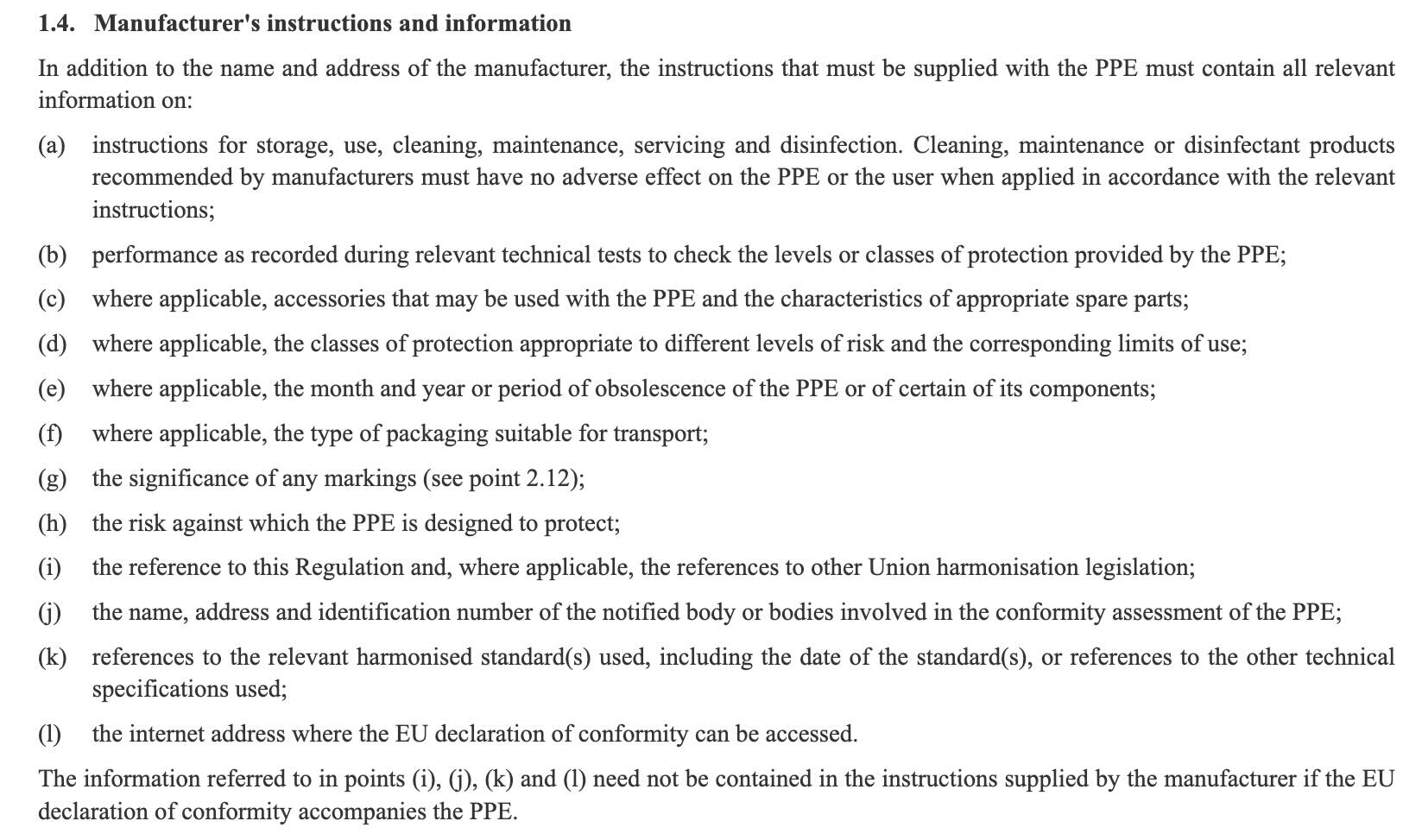
Most CE marking regulations and directives mandate the inclusion of instructions that must generally be provided together with the product. While instructions are sometimes printed on the product or packaging, it is common to provide instructions in the form of a printed user manual.
The exact information and wording of the instructions must often be decided by the manufacturer, which makes it challenging to know if you got it right or not.
Example: PPE Regulation > Annex II > 1. GENERAL REQUIREMENTS APPLICABLE TO ALL PPE
Technical Documentation
The technical documentation can be seen as the backbone of the entire CE marking process. It includes everything that defines the product’s design, functionality, and compliance status.
In practice, the technical documentation is generally a set of files that cover the following areas:
1. Product description
2. Technical drawings
3. Bill of materials
4. Product and packaging label artwork
5. Copies of instructions
6. Test reports
7. Declaration of Conformity copy
8. Calculations and other relevant data
9. Risk assessment
Technical Documentation Sample
EC-Type Examination Certificate
Certain products require the involvement of a Notified Body before being sold in the European Union. This is the case for certain PPE categories and medical device classes, among other product types.
The role of the Notified Body is generally to act as a market gatekeeper. They review the DoC, instructions, and other documentation – and may also be involved in the testing process.
What can come out of this process is an EC-Type Examination Certificate issued by the Notified Body, declaring that a certain product complies with specific CE marking requirements.
Overview
| Document | Issued by | Provided to* |
| Declaration of Conformity | Manufacturer | 1. Market surveillance authorities 2. Marketplaces 3. Retailers |
| Test report | Testing lab | 1. Market surveillance authorities 2. Marketplaces 3. Retailers |
| Instructions | Manufacturer | Customers |
| Technical documentation | Manufacturer | Market surveillance authorities |
| EC-Type Examination Certificate | Notified body | 1. Market surveillance authorities 2. Marketplaces 3. Retailers |
*The table specifies common cases for the types of entities for which certain CE marking documentation may need to be provided.
FAQ
Can we CE mark a product without these documents?
No, these documents are an integral part of the CE marking process. You could face a recall or be fined if you lack sufficient documentation, even if your product is technically safe and compliant.
How do I know what CE marking documentation is required for my product?
The first step is to identify applicable EU regulations and directives for your product. Once this is done, you can identify the documentation requirements, which are often summarised in the Annexes.
Who is responsible for preparing the CE marking documentation?
The manufacturer is generally responsible for issuing the bulk of the CE marking documentation.
What responsibility do importers have for obtaining CE marking documents?
While importers generally don’t issue documentation to support CE marking, they must obtain and verify documentation from the manufacturer.
This can only be done if the importer has a profound understanding of what is required and the information that must be provided in the documentation. Accepting documents at face value can be disastrous.
What should we do with the CE marking documentation?
The CE marking documentation can be requested by the customs authorities upon arrival. Failing to provide the DoC, test reports, and other documentation can result in the goods being seized and destroyed without compensation.
Further, EU market surveillance authorities can request CE marking documentation months or even years later.
Amazon and other marketplaces are also verifying documents supporting CE marking. Failing to provide the requested documentation often results in recalls and removals.






















.png)
.png)
.png)