Compliance Gate Platform
Find Compliance Requirements for Your Products in Minutes
- Create certificates, labels & book testing
- Get access to personal support

Affordable:Get access from only $199 per year

Save hours:Find product requirements in 2 min instead of 5 hours

User-friendly:Created for non-experts and busy professionals
Find product requirements in 2 minutes
This is also included
Project Management
Use the project modules to break down key requirements into actionable task lists.
- US: CPSIA / General use products
- EU: GPSR / CE Marking
- UK: GPSR 2005 / UKCA Marking
Create Product Certificates
Access a library of templates to create mandatory product certificates and declarations
- CPC and GCC templates (US)
- Declaration of Conformity (EU / UK)
- Technical Documentation (EU / UK)
- Instructions (EU / UK)
- FCM Declaration of Compliance (EU / UK)
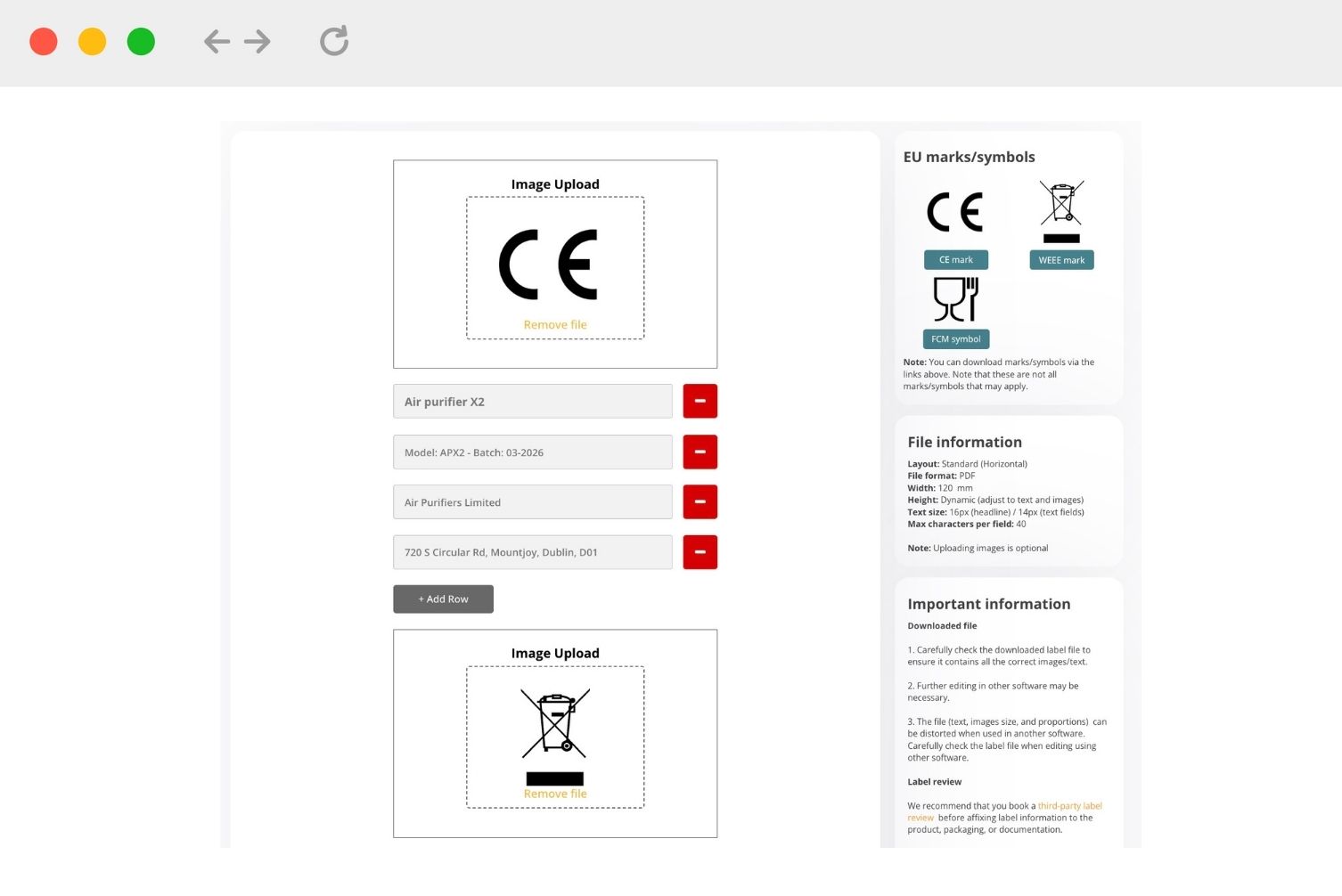
Label Templates
Create product and packaging labels using ready-made layouts and templates
- Product labels
- Packaging labels
- Download compliance marks
- Download as PDF
Other Features
Ask questions via support tickets (View)
Questions about compliance requirements? Submit a ticket and we reply in 1-2 business days.

Ask up to 5 questions per ticket
How is the platform updated? (View)
We monitor US, EU, UK & CA sources each month. You will also receive a monthly report.

Monthly updates included
Covered Products (Examples)
Below are examples of product categories and materials the platform covers to some extent.
General Consumer Products
Many products do not fall under product-specific regulations or directives. However, the platform covers regulations, chemical restrictions, labelling and certification requirements for general consumer products sold in the US, EU, UK, and Canada.
Examples: Accessories, bags, pet products, household decor, artworks, phone cases, laptop cases, stationery items, leather goods
Product Examples

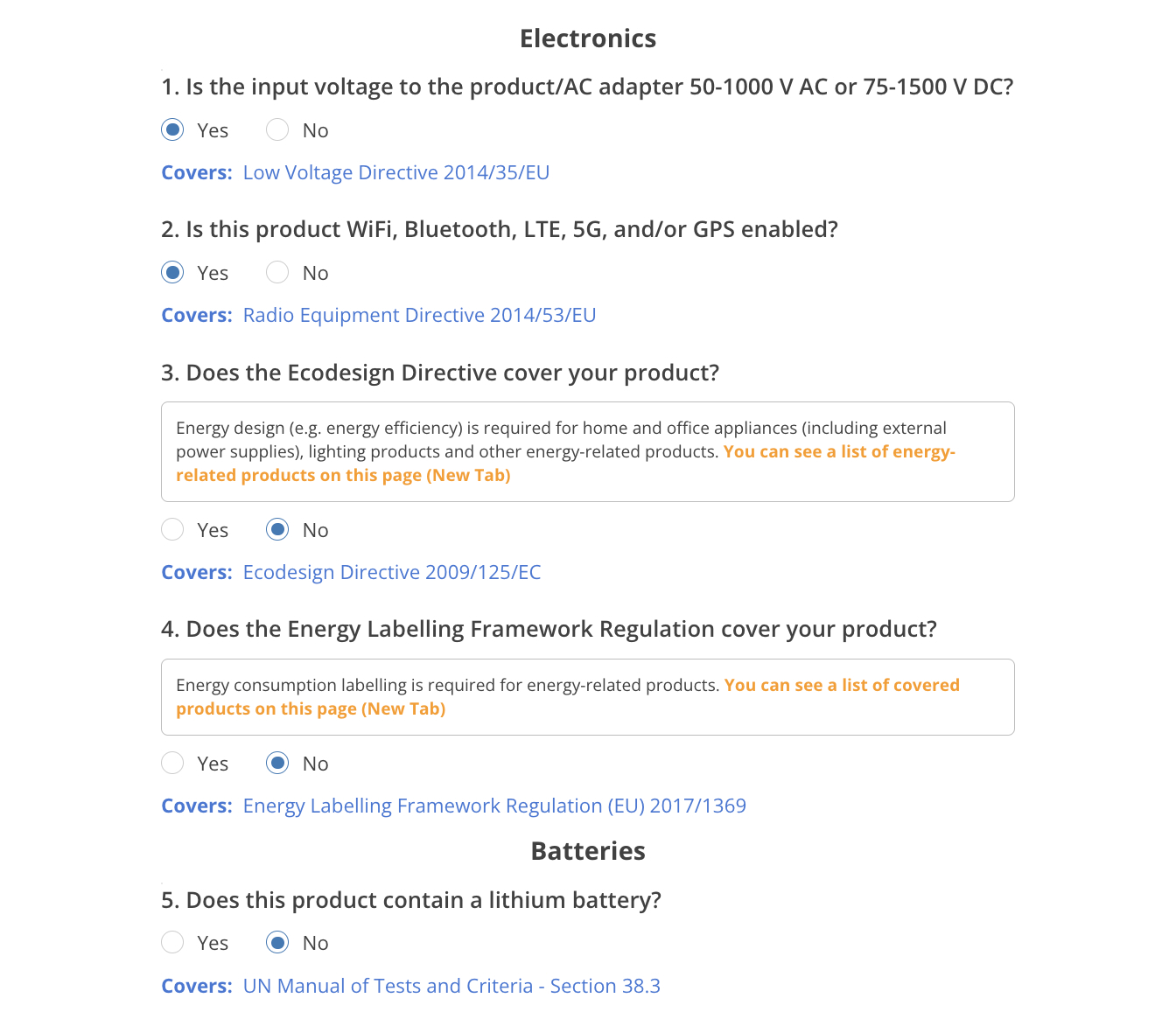
Electronics
.png)
.png)
.png)

Toys
.png)
.png)
.png)


Children’s Products
.png)
.png)
.png)


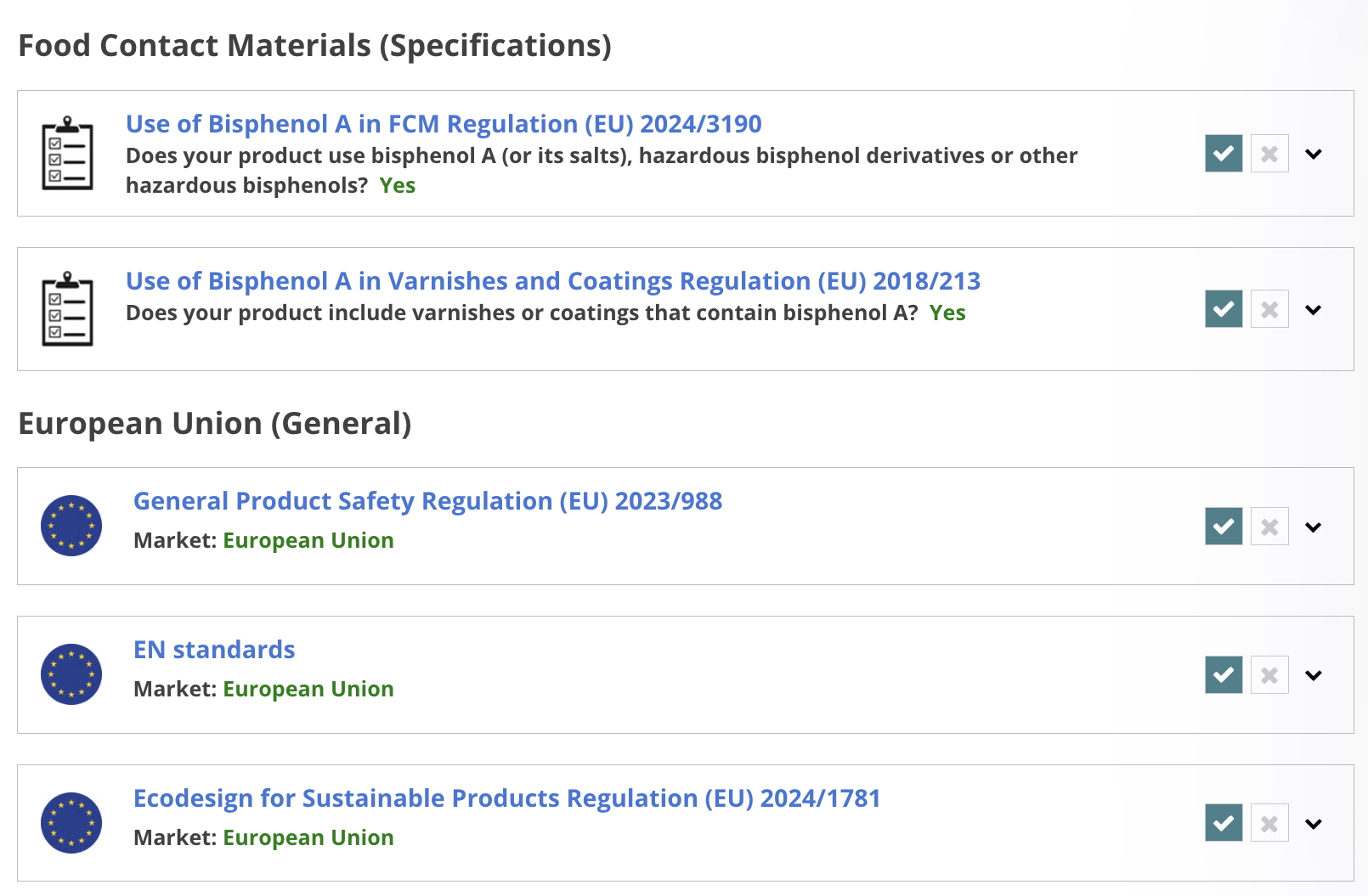
Food Contact Materials
.png)
.png)
.png)


Training Equipment
.png)
.png)

Product Packaging
.png)
.png)
.png)


Textiles
.png)
.png)
.png)


Clothing
.png)
.png)
.png)


Law Labels
.png)

Carpets and rugs
.png)
.png)
.png)

Magnets
.png)

Jewelry
.png)
.png)

Precious Metals
.png)

Teats and Soothers
.png)
.png)

Footwear
.png)
.png)
.png)

PPE
.png)
.png)

Furniture
.png)
.png)
.png)

Mattresses
.png)
.png)
.png)

Candles
.png)

Art Materials
.png)

Measuring Instruments
.png)
.png)

Machinery
.png)
.png)

Batteries
.png)
.png)
.png)
Energy Related Products
.png)
.png)

DoE energy-efficiency standards
.png)

AI Devices
.png)

Wood and plant products
.png)
.png)
.png)

Construction Products
.png)
.png)

Gas Appliances
.png)
.png)

Medical Devices
.png)
.png)
.png)


Recreational Crafts
.png)
.png)

Cosmetic Products
.png)
.png)


Aerosol Dispensers
.png)
.png)

Biocides
.png)
.png)

Ecolabel
.png)

Consumer Rights Directive
.png)
Pesticides
.png)

Pest Control Products

Fluorinated greenhouse gases
.png)
.png)

Environmental claims
.png)
National Organic Program
.png)

Occupational Safety and Health
.png)

Workplace Products

Can’t find your product?
The Compliance Gate Platform can be useful for most consumer products, even if not mentioned above. Furthermore, the listed modules often cover many different product types (i.e., EU machinery covers e-bikes).
However, the platform does not cover supplements, pharmaceuticals, and food and beverages.
You can also ask!
Still not sure if the platform is relevant to your product?
Feel free to book a call or write to us via the contact form
Covered regulations/requirements
Notice that the platform only covers the items listed in the US / EU / UK / CA Databases linked below:
-
Features
-

Compliance Requirements List (View)
-
>
.png)
United States
-
>
.png)
European Union
-
>
.png)
United Kingdom
-
>

Canada

-
-

Document templates
-

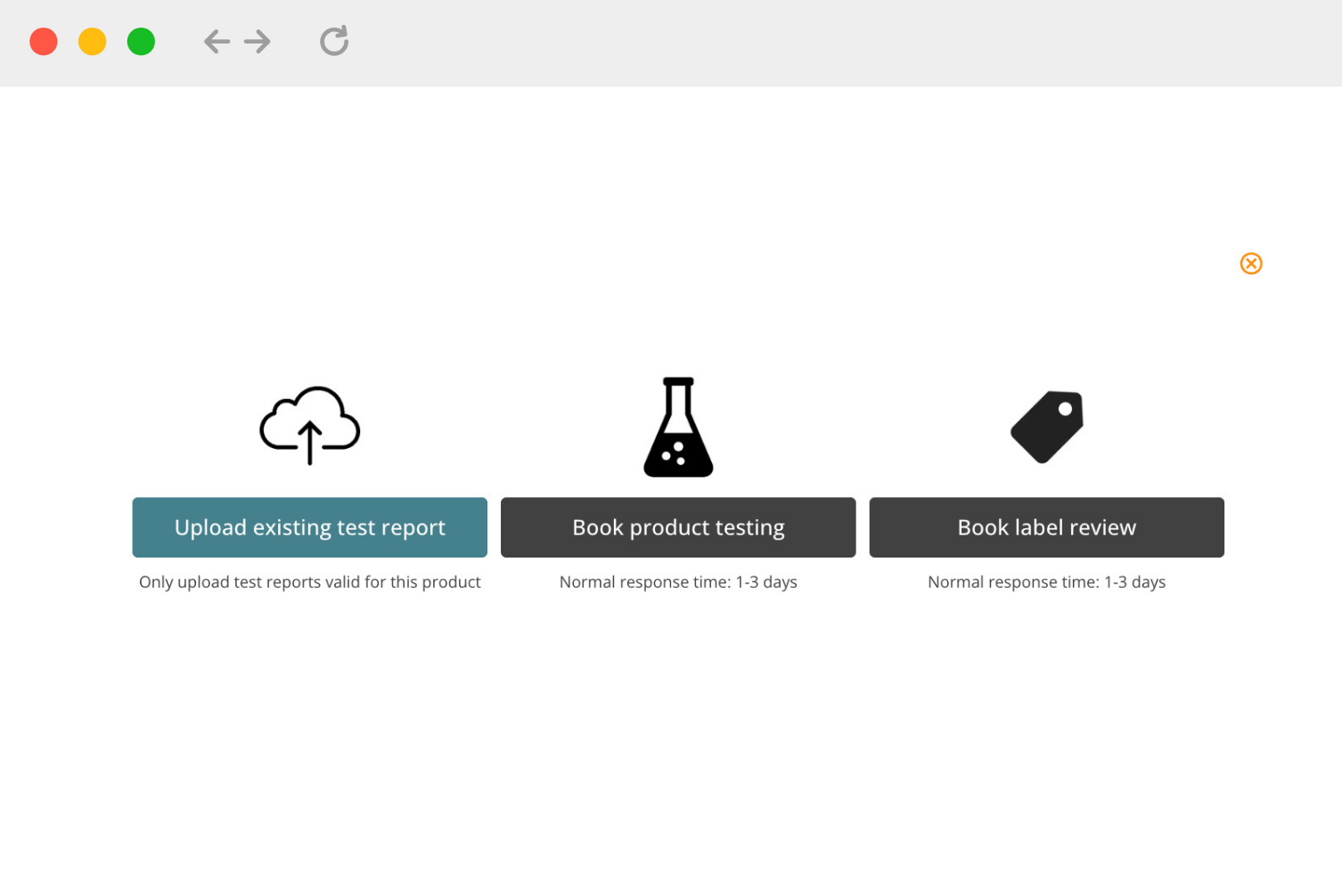
Book lab testing
-
Other
-

Monthly Compliance Report
-
Support tickets
-

Users
-
-
Features
-

Compliance Requirements List (View)
-
>
.png)
United States
-
>
.png)
European Union
-
>
.png)
United Kingdom
-
>

Canada

 Full Access
Full AccessUnlimited use
-
-
-

Document templates

Book lab testing
Other

Monthly Compliance Report


Support tickets

Users
1 user
-

Premium
$299per year
Includes full access to all platform features for 12 months
-
Features
-

Compliance Requirements List (View)
-
>
.png)
United States
-
>
.png)
European Union
-
>
.png)
United Kingdom
-
>

Canada

 Full Access
Full AccessUnlimited use
-
-

-

-

Document templates

-

Book lab testing

-
Other
-

Monthly Compliance Report

-

Support tickets
-

Users
1 user
-

Business
$499per year
Full access for 12 months, plus 10 support tickets and 3 users
-
Features
-

Compliance Requirements List (View)
-
>
.png)
United States
-
>
.png)
European Union
-
>
.png)
United Kingdom
-
>

Canada

 Full Access
Full AccessUnlimited use
-
-

-


Document templates


Book lab testing

Other

Monthly Compliance Report


Support tickets
10 x Tickets

Users
3 users
Included in every plan (at no additional cost)
Personal onboarding session ($129 value)
We will help you add your first product and show how to use the platform (30-min video call)

No additional cost
ComplianceGate Academy ($79 value)
Instant access to tutorials and videos demonstrating how you can use the platform features.

No additional cost
Can I cancel at any time?
Yes, and we will send 2 email reminders before any renewal
Disclaimers
General Disclaimer: The Compliance Gate Platform is not a lawyer or a law firm and does not engage in the practice of law or provide legal advice or legal representation. All information, software, services, and support provided on the site are for informational and self-help purposes only and are not intended to be a substitute for professional legal advice. Use of the Compliance Gate Platform and this site is subject to our Terms of Service.
Compliance Requirements List (CRL) Disclaimer: Each CRL is created based on user inputs and contains CRL entries (based on the source version available at the time of writing). CRL entries are provided for general informational purposes only and do not contain legal advice. We do not guarantee the completeness or accuracy of CRL entries, nor do not claim to cover all regulations or requirements that may apply to a particular product. A newer version of the source that the CRL entry is based on may exist. You can access the relevant source via the blue button the links in the source footer. Select the latest version of the relevant source/s. You must ultimately take action based on the requirements in the latest version of the relevant legislation, guidance pages or other sources. You should not take action based on the CRL entry alone, as it does not contain the entire source text.
Note: We only cover the items listed in these documents:
• US Database
• EU Database
• UK Database
• CA Database
Templates Disclaimer: Templates are based on specified parts of legislation and/or official guidance documents (based on the source version available at the time of creation). Templates do not constitute legal advice. We do not claim to provide templates for all documents or labels that may apply to a particular product. A newer version of the source that the template is based on may exist. You must ultimately create/affix/submit documents and labels based on the latest version of the relevant legislation, guidance pages or other sources.
Updates Disclaimer: We monitor certain legislation texts and other sources in certain countries/markets. We only monitor regulations and updates as these are published in legislation databases and may report on changes after they have entered into force and/or apply. We do not guarantee that we ‘catch’ every single new or updated compliance requirement that may apply (including covered product modules) or that all CRLs/templates are up to date. Certain features (i.e., templates and courses) are only reviewed during a yearly audit. You must ultimately take action based on the requirements in the latest version of the relevant legislation, guidance pages or other sources.
Note: We only monitor sources listed in these documents:
• US Database
• EU Database
• UK Database
• CA Database
Support Tickets Disclaimer: Support tickets allow you to ask questions about product compliance requirements. Support is generally limited to requirements in the CRL database and only to the extent that questions are clearly addressed in official source texts. We do not provide legal advice, interpretations, engineering/technical advice, or confirm applicable regulations or standards for specific products. We cannot “confirm” or “approve” a product as fully compliant or approve user-created labels and documents. You must ultimately take action based on the requirements in the latest version of the relevant legislation, guidance pages or other sources.
Other information
1. Read the Feature Overview to learn more about the features of the Compliance Gate Platform, and their limitations and risks.
2. Technical problems can occur when creating, saving or downloading compliance requirements lists and other files due to extenuating circumstances like software bugs or internet issues. This can result in the downloaded document being incomplete or otherwise incorrect.
3. The platform does not cover or provide access to product standards.
Product Tour Video
Documentation
Feature Overview
This document explains the use case, data sources, limitations, and risks of the following Compliance Gate Platform features:
- Compliance Requirements Lists
- Project Modules
- Document/Label Templates
- Support Tickets
- Lab Testing / Other Third-Party Services
- Monthly Compliance Report / Source Monitoring

US Database
Compliance Requirements List (CRL)
- We only cover the regulations/requirements listed in this document.
- We specify which regulations/requirements are monitored and provide the URL. Not all entries in the database have sources that are actively monitored.
- You can see the current status of each entry and the source version they are based on.
Document Templates
- We only cover document templates listed in this document.
- You can see the current status of each document template and the source version they are based on.

EU Database
Compliance Requirements List (CRL)
- We only cover the regulations/requirements listed in this document.
- We specify which regulations/requirements are monitored and provide the URL. Not all entries in the database have sources that are actively monitored.
- You can see the current status of each entry and the source version they are based on.
Document Templates
- We only cover document templates listed in this document.
- You can see the current status of each document template and the source version they are based on.

UK Database
Compliance Requirements List (CRL)
- We only cover the regulations/requirements listed in this document.
- We specify which regulations/requirements are monitored and provide the URL. Not all entries in the database have sources that are actively monitored.
- You can see the current status of each entry and the source version they are based on.
Document Templates
- We only cover document templates listed in this document.
- You can see the current status of each document template and the source version they are based on.

CA Database
Compliance Requirements List (CRL)
- We only cover the regulations/requirements listed in this document.
- We specify which regulations/requirements are monitored and provide the URL. Not all entries in the database have sources that are actively monitored.
- You can see the current status of each entry and the source version they are based on.
Document Templates
- We only cover document templates listed in this document.
- You can see the current status of each document template and the source version they are based on.
Data Management & Security
This guide covers the following:
- Security
- How to remove your product data
- Subscription expiry
- Terminate account
- Backups
Compliance Gate Platform FAQ
General
Feature Overview
The Feature Overview document explains the use case, data sources, limitations, and risks of the following Compliance Gate Platform features:
- Compliance Requirements Lists
- Project Modules
- Document/Label Templates
- Support Tickets
- Lab Testing / Other Third-Party Services
- Monthly Compliance Report / Source Monitoring
Please read this document and this FAQ carefully.
Product Database
- Startup: 200
- Premium: 200
- Business: 500
- Enterprise: 2,000
Usage rights
- Startup: Your organisation
- Premium: Your organisation
- Business: Your organisation
- Enterprise: Your organisation / Third parties (Info)
What is the purpose of the Compliance Gate Platform?
The purpose of the Compliance Gate Platform is to help businesses manage practical aspects of the compliance process. This is an example of a workflow:
1. Create a compliance requirements list to find relevant compliance requirements
2. Access the latest version of the relevant legislation, guidance document, or other sources to identify specific product safety, labelling, documentation, and other requirements.
3. Book third-party lab testing
4. Create declarations and certificates using the templates
5. Create product and packaging label files using the label creator
Support tickets
Throughout this process, you can ask questions to our team by submitting support tickets.
Use case principle
The Compliance Gate Platform is built to streamline parts of the compliance process that can be time consuming and tedious. However, the platform functions as a navigation tool rather than a definitive source for product compliance requirements. As such, it is important to always take action based on the latest version of the relevant legislation, guidance documents or other sources.
What are some benefits of the Compliance Gate Platform?
Here are some of the key benefits of using the Compliance Gate Platform:
Launch new products: Find compliance requirements, create mandatory documents, create labels, and book testing.
Discover new requirements: You can also use the Compliance Gate Platform to find requirements that you may not be aware of already.
Save time: Researching compliance requirements online can take hours, and you may not even know where to start. With our platform, you can find requirements in minutes, while also having access to ready-made templates, easy to use label layouts, and access to lab testing companies.
Wide product range: You can combine modules to research requirements for a wide range of consumer, commercial, and industrial products. Are you selling battery-powered electronic toys? Then select the modules for electronics, batteries, and toys.
Is the Compliance Gate Platform easy to use?
The Compliance Gate Platform was created with a clean UI and accessibility in mind. Our ambition is to make the process as direct and streamlined as it can be.
That being said, we don’t claim to make the product compliance process simple or easy. Ultimately, you must follow the requirements in the latest version of the relevant legislation, guidance documents, or other sources. Such requirements can be complex.
The Compliance Gate Platform can be a helpful tool for navigating requirements and completing parts of the process, but it does not provide shortcuts or ways to avoid compliance requirements.
Does the Compliance Gate Platform only cover consumer products?
No, the platform covers many products that are not consumer products. Examples include construction products, machinery, protective equipment, and medical devices.
Why are there so many feature limitations and risks?
There is no established standard for what a product compliance software can or cannot do. It’s therefore essential that we are transparent and clearly explain how the platform and features were created.
This also means that we must be transparent and carefully disclose the risks and limitations of the platform and its features. More information can be found in the Feature Overview linked above.
Does the platform cover all regulations and requirements that may apply to a certain product?
No, we do not have a complete database of all existing regulations, directives, standards, rules, or other compliance requirements that may apply to a certain product.
The products listed on this page indicate that we cover at least some compliance requirements that relate to these products.
You can find a list of covered items here:
Do you guarantee that my products will become compliant and safe if we use the platform?
The Compliance Gate Platform is not capable of approving or confirming if a product is fully compliant. The platform depends entirely on user inputs to create compliance requirements lists and use other features.
Which products do you cover?
You can see a list of product categories on this page. The products listed on this page indicate that we cover some compliance requirements that relate to these products.
Which sources do you use?
The Compliance Gate Platform database is based on publicly available information from official sources in the US, EU, UK, and Canada. Here are some examples:
- europa.eu
- EUR Lex
- eCFR
- US government agency websites (e.g., CPSC, FCC, FDA, DOE, FTC, and USDA)
- gov.uk
- legislation.gov.uk
- laws-lois.justice.gc.ca
Does the platform provide access to standards?
The Compliance Gate Platform does not cover product standards that are not publicly available. Hence, it does not cover the contents of EN standards, ISO standards, ASTM standards, ANSI standards, or UL standards.
Which countries or markets do you cover?
We only cover compliance requirements in the following countries and markets:
- United States
- European Union
- United Kingdom
- Canada
Do you cover US state requirements?
We provide general information about certain areas of US state regulations and some (but not all) individual US state regulations. Further, some are not monitored.
Do you cover European national requirements?
We do not cover national legislation of EU member states (including legislation based on EU directives).
Do you cover Canadian provincial requirements?
We only cover some (but not all) Canadian provincial regulations.
Do you cover customs and tax regulations?
No, the Compliance Gate Platform does not cover regulations that relate to customs procedures or taxes.
We only focus on requirements relevant to the product and its packaging.
Compliance Requirements Lists
What is a Compliance Requirements List?
1. Compliance Requirements Lists (CRL) are created based on the following user inputs:
- Market
- Module/s
- Specifications and parameters
2. By creating a requirements list, you can find relevant product regulations and other requirements.
3. Each Compliance Requirements List (CRL) contains Compliance Requirements List (CRL) entries.
Note: The modules and specifications are generally based on the scope defined in the relevant legislation. When this is not the case, we search for guidance documents that provide a scope. However, a regulation or other subject may still apply to products/materials/scenarios that fall outside of what is defined in a particular module or specification. Further, we do not claim that a module or specification covers all regulations or other requirements that belong to the covered subject (i.e., toys or PPE).
Which regulations, directives, and other requirements are covered?
The Compliance Requirements List database only covers US, EU, UK and Canada regulations listed in these documents:
What is a CRL entry?
1. Each CRL entry contains an overview of the contents of the covered regulation, directive, document, or other compliance topic. These may, for example, list the following:
- Documentation
- Labelling
- Substance restrictions
- Testing requirements
- Source/s
Note: Most CRL entries contain icons that can help you identify specific areas. For example, articles/parts specific to labelling requirements may have a label icon. Note that the icon is only added based on the title of the relevant article/part. Other articles/parts may also contain such requirements, even if there is no icon indicating this.
2. CRL entries serve the following purposes:
- It provides a general overview of requirements
- It helps you navigate to relevant articles/annexes/regulations/parts of the covered source/s (i.e., where to find labelling requirements)
- It guides you to relevant legislation, guidance pages, and other sources
3. A CRL entry does not contain the entire legislation, guidance document or other source. As such, it does not contain all the requirements or details found in the relevant source.
4. CRL entries are provided for general informational purposes only and do not contain legal advice.
How do you create CRL entries?
1. We take the following steps when writing a CRL entry:
- Select the regulatory text and/or other sources (e.g., guidance page)
- We create an index of articles/regulations/parts/guidance page items
- We add navigational icons, images, template buttons, and other relevant information
2. We have a standard CRL entry template that can also be adjusted depending on the contents and structure of the relevant regulation, guidance page or other source covered.
Note: We generally only list individual articles/regulations/parts/guidance page items for manufacturers, importers, and/or other entities that must actively manage the compliance process. Items relevant to government bodies, third-party service providers (e.g., notified/approved bodies), marketplaces, fulfilment centres, and other entities are generally only listed as chapters.
How should we use CRL entries?
1. A CRL entry contains an overview of the contents of the covered regulation, directive, document, or other compliance topic. It serves as a first introduction to the covered subject.
2. You can access the relevant source by clicking on the blue button below the introduction, or follow the links in the source footer. Ensure that you select the latest version of the relevant legislation, guidance pages or other sources.
3. You must ultimately take action based on the requirements in the latest version of the relevant legislation, guidance pages or other sources. You should not take action based on the CRL entry alone, as it does not contain the entire source text.
Sources and versions
1. Each CRL entry is based on legislation texts and/or official guidance pages/documents.
2. We primarily use data from the following sources:
- europa.eu
- EUR Lex
- eCFR
- US government agency websites (e.g., CPSC, FCC, FDA, DOE, FTC, and USDA)
- gov.uk
- legislation.gov.uk
- laws-lois.justice.gc.ca
3. Each CRL entry is based on the source version available at the time of writing (more recent versions of the source may be available).
4. You can find information about the CRL entry versions, monitored sources, and how we manage updates in the following documents:
Limitations and risks
You can find a list of limitations and risks related to Compliance Requirements Lists in this document.
How can we benefit from creating a Compliance Requirements List?
It can take hours of research to find information about relevant product compliance requirements in legislation databases, such as EUR Lex or the eCFR. This also assumes that you even know where to look for information in the first place.
Our platform makes it possible to create Compliance Requirements Lists in a far shorter time frame. The time saving can be immense, especially if you are not already familiar with product compliance requirements.
That said, it is important to understand that researching relevant requirements is only the first step in the process. You must ultimately take action based on the latest version of the legislation, guidance page or other source that a CRL entry is based on.
Do I need to create a Compliance Requirements List for each product?
Yes, you generally need to create a Compliance Requirements List for each product, as the requirements depend on product specific parameters.
Do I need to create a Compliance Requirements List for each country/market?
Yes, you need to create a unique Compliance Requirements List for each product and market. For example, if you sell the same product in the US and the EU, then you must create two separate Compliance Requirements Lists.
Do Compliance Requirements Lists cover all requirements applicable to our product?
Our database is limited to the items listed in these documents:
We do not claim to cover all existing regulations that may apply to a certain product, category, or industry. Further, we do not claim to cover all regulations and other compliance requirements that apply to products.
Do the modules/product examples cover all applicable requirements for that category?
No, we do not claim that the modules cover all requirements that may exist for a particular product category. For example, the EU electronics module covers regulations and directives in our database that are specifically relevant to electronic products in the European Union.
But, there may still be other regulations and directives that apply to electronics that are not covered by our database. This is also why we state that the product examples provided on the website are examples of product categories and materials the platform covers to some extent.
Do CRL entries contain all requirements of the covered regulation/directive?
The CRL entries do not contain the entire legislation source text. Hence, they do not cover every requirement, scenario, or piece of information present in the source text.
1. A CRL entry serves as an introduction that provides a general overview (available at the time of writing) relevant to manufacturers or other entities that actively manage the compliance process. While it serves as a first introduction, it is not intended as an exact step-by-step guide for how compliance can be achieved. It would not be possible to write a summary that provides a specific roadmap for every single entity, product, or scenario.
2. A newer version of the legislation, guidance page or other source that the CRL entry is based on may exist. This means that articles, regulations, parts, or other requirements may not be present in the CRL entry.
3. You must ultimately take action based on what is written in the latest version of the relevant legislation, guidance page or other source.
Can you guarantee that all CRL entries are free of errors?
We do our best to fact-check the CRL entries, but due to the amount of information and that the sources may be updated after publication, we cannot guarantee that the CRL entries are completely free of error.
You must ultimately take action based on what is written in the latest version of the relevant legislation, guidance page or other source.
At what stage should I create a compliance requirements list?
You should create a Compliance Requirements List as early as possible in the product development process. This is because the list can direct you to relevant product regulations, which contain information about safety, labelling, and testing requirements that have a direct impact on the design and functionality of the product.
Do the Compliance Requirements Lists include standards?
Product standards are sometimes referenced (directly or indirectly) if they are referenced in a regulation, directive, or compliance requirement. That said, we do not have access to standards, and their contents are therefore not included in the CRL entries.
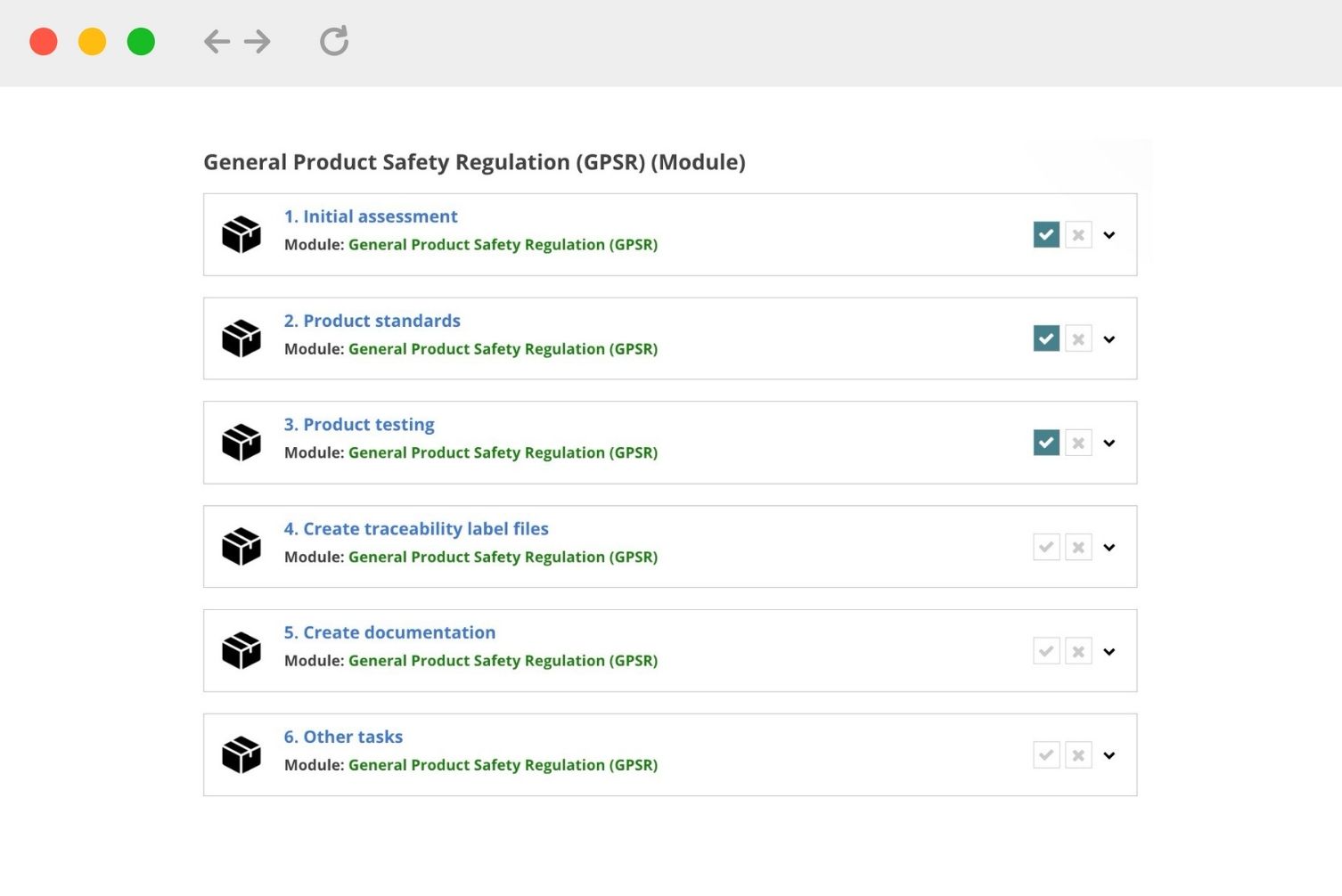
Project Modules
What is a project module?
1. Project modules break down certain compliance requirements into actionable steps.
2. You can add project modules when creating a Compliance Requirements List.
Which project modules are included?
You can find a list of US, EU, UK, and Canada project modules (and the data and versions these are based on) in these documents:
How do you create project modules?
1. We create project modules based on selected requirements for manufacturers/producers under certain regulations or groups of regulations.
2. Note that some project modules are more strictly based on a single regulation (i.e., EU GPSR) while other project modules provide a more generalised procedure based on requirements common to many products (i.e., CE marking).
How should I use a project module?
1. A project module provides a list of tasks that correspond to key requirements within certain regulations or other areas of product compliance. The project modules can help you keep track of progress.
2. That said, you must ultimately take action based on the latest version of the relevant regulation, guidance page, or other source. You should not take action based on the project module alone for the following reasons:
a. Project modules may not contain all tasks that must be completed for a certain product (other regulations/requirements not covered by project modules may still apply).
b. Project modules may not contain all tasks that must be completed to comply with the regulation/requirement these are based on (for example, there are other tasks under GPSR that can apply in some cases, but are not covered by the project module).
c. Project modules are only reviewed and subject to updates after an annual audit in October each year. As such, the project modules can be outdated if the regulations, guidance pages, or sources are updated.
Sources and versions
1. Some project modules are based on specified parts of legislation texts and/or official guidance pages/documents.
Note: Not all project modules are based on a specific source. Certain project modules are only intended to provide a generalised breakdown of selected tasks.
2. We primarily use data from the following sources:
- europa.eu
- EUR Lex
- eCFR
- US government agency websites (e.g., CPSC, FCC, FDA, DOE, FTC, and USDA)
- gov.uk
- legislation.gov.uk
- laws-lois.justice.gc.ca
3. Each project module is based on the source version (or other information) available at the time of creation (a more recent version of the source may be available).
4. You can find information about the versions, sources, and how we manage updates in the following documents:
Limitations and risks
You can find a list of limitations and risks related to the Project Modules in this document.
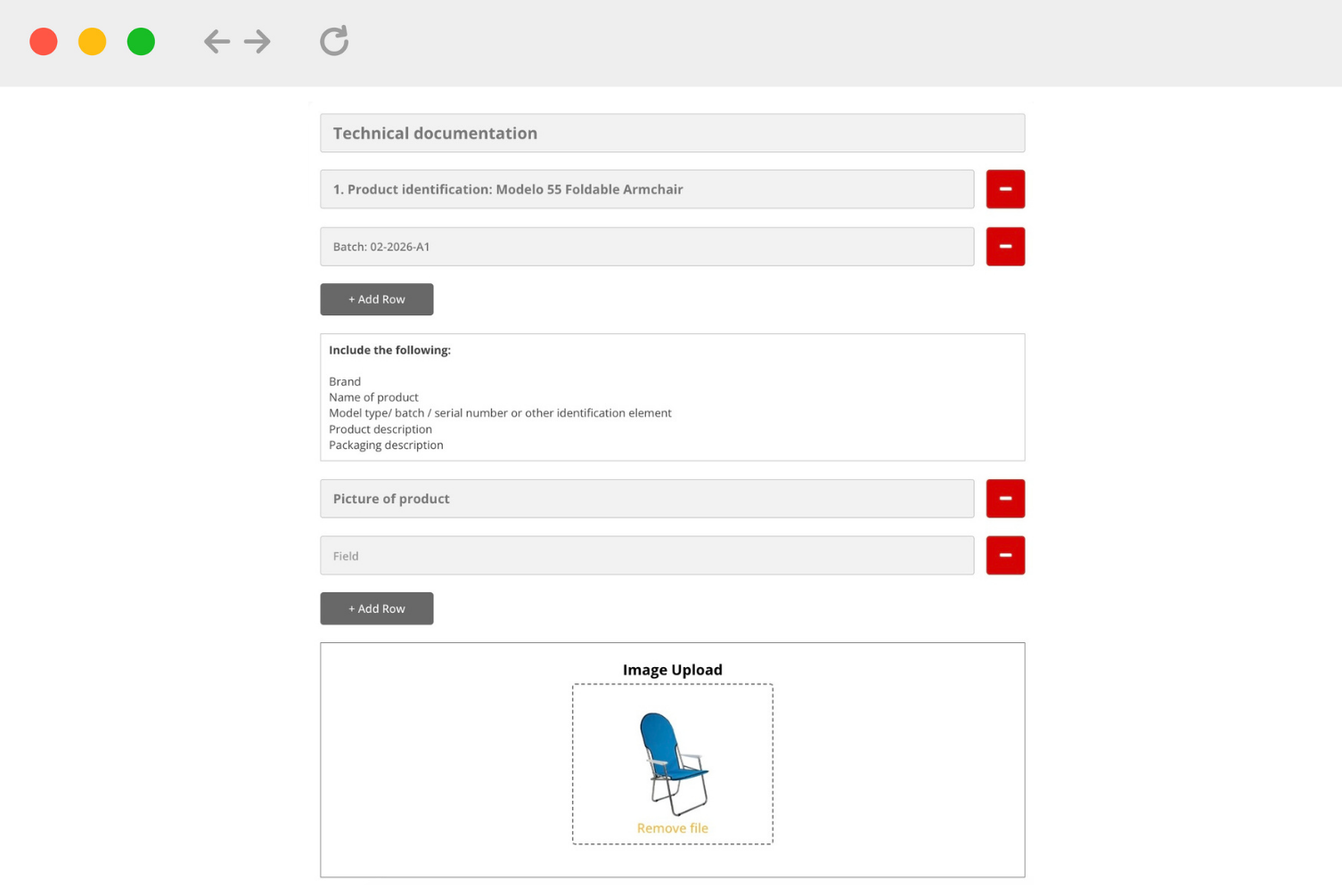
Document / Label Templates
What is a document template?
1. Certain legislation mandates the creation of declarations, certificates, technical documentation and other documents. Such documents can be requested by national authorities and marketplaces, such as Amazon.
2. The document templates are based on specified parts of legislation and/or official guidance documents.
What is a label template?
1. Certain legislation sets requirements concerning marking and labelling requirements. Labels can, depending on the specific placement requirements, be printed on product, packaging, and/or accompanying documents.
2. The label templates are based on specified parts of legislation and/or official guidance documents.
Which templates are included?
You can find a list of US, EU, UK, and Canada templates (and the data and versions these are based on) in these documents:
How do you create document and label templates?
1. We base document templates on what is written in legislation and/or guidance documents.
2. For example, the EU Declaration of Conformity document template is based on the EMC Directive 2014/30/EU > ANNEX IV.
3. Label templates are only based on the specified parts of a certain legislation.
How should we use document/label templates?
1. Start by selecting a template relevant to your product.
2. You must carefully read the instructions in the right sidebar and follow these guidelines:
- You may need to make edits to the template before adding information.
- Open the source link and select the latest version of the relevant legislation, guidance page or other source
- Ensure that you fill in a template based on the latest available version of the source/s
Note: The templates are only updated during an annual audit in October. As such, the templates may be outdated if the source (legislation or guidance document) is updated after the annual audit. It is therefore important to check the latest source version.
Note: You must ultimately create/affix/submit documents and labels based on the requirements in the latest version of the relevant legislation, guidance pages or other sources. Note that label placements can be conditional. Furthermore, the exact wording used in documents, instructions, warnings, and other files can be open-ended and not clearly specified in legislation or other sources.
Note: Definitions in legislation may provide information about the particular economic operators and product details that may need to be present in documents and labels.
3. Add information to the fields and upload image files (when necessary). Note that some documents can only be issued if there is other supporting documentation, such as test reports.
4. Download a PDF copy of the document or label after completion and ensure that you maintain backups at all times.
Document note: Certain regulations require that documents be maintained for a certain period of time (e.g., 10 years after you have placed a product on the market). Further, some documents must also be printed and signed.
Label note: Further editing in other software may be necessary. We also recommend that you book a third-party label review before affixing label information on the product, packaging, or documentation.
Sources and versions
1. Each document and label template is based on specified parts of legislation texts and/or official guidance pages/documents.
2. We primarily use data from the following sources:
- europa.eu
- EUR Lex
- eCFR
- US government agency websites (e.g., CPSC, FCC, FDA, DOE, FTC, and USDA)
- gov.uk
- legislation.gov.uk
- laws-lois.justice.gc.ca
3. Each document and label template entry is based on the source version available at the time of creation.
4. You can find information about the document template versions, sources, and how we manage updates in the following documents:
Limitations and risks
You can find a list of limitations and risks related to the Document/Label Templates in this document.
Do you provide templates for all documentation and labels required in the US, EU, UK, and Canada?
No, we only provide the templates listed here:
We do not claim to provide templates for all documentation/certification/labelling requirements in existence in the mentioned markets, or that may apply to a particular product.
How do you keep the document and label templates up to date?
The templates are only updated during an annual audit in October. As such, the templates may be outdated if the source (legislation or guidance document) is updated after the annual audit. It is therefore important to check the latest source version.
The template must be adjusted based on the latest version of the relevant article, annex, regulations, part, or guidance document before it is filled in.
What can happen if I don’t have the mandatory compliance documents?
Lacking the mandatory product certificates and other compliance documents can result in a recall, or that your products are removed from marketplaces such as Amazon.
How do we make sure that the label files are correct?
You can use the label templates as a starting point, but you must ultimately create label files based on the latest versions of all relevant regulations, guidance documents, and other sources.
Note that a product can be subject to many regulations which all set labelling requirements.
Support tickets
What is a support ticket?
Support tickets allow you to ask questions about US, EU, UK, and Canada product compliance requirements directly to our support team. We are available from Monday to Friday.
- You can submit a support ticket listing up to 5 questions.
- Each support ticket can only be dedicated to a single product and market.
- We can generally only provide support related to requirements in the CRL database
How can I use support tickets?
Sources and versions
1. We can only respond based on what is written in regulation texts, guidance pages, and other official sources. Here are some examples of sources we use:
- europa.eu
- EUR Lex
- eCFR
- US government agency websites (e.g., CPSC, FCC, FDA, DOE, FTC, and USDA)
- gov.uk
- legislation.gov.uk
- laws-lois.justice.gc.ca
2. This also means that we can only address questions to the extent that these are addressed in the regulation texts, guidance pages, and other official sources. Bear in mind that the sources do not address every possible scenario or question.
3. Note that we can also provide guidance based on our own experience. However, this is limited to practical aspects of the process (e.g., how to submit samples to testing companies).
Limitations and risks
You can find a list of limitations and risks related to the support tickets in this document.
How many questions can we ask per support ticket?
1. You can submit a support ticket listing up to 5 questions.
2. Each support ticket can only be dedicated to a single product and market.
3. You can ask questions related to information in a Compliance Requirements List.
What kind of questions can we ask?
1. You can ask questions about information in the generated compliance requirements lists:
- Product regulations and directives
- Certificates and documents
- Labelling requirements
- Testing requirements
2. You can ask questions about practical areas of product compliance, such as:
- Label file formats
- Lab testing costs
- Supplier vetting
3. Request help with lab test bookings and other third-party services
4. Request feedback on test reports and documents obtained from your suppliers
How many tickets are included?
The number of support tickets depends on your plan:
- Startup: None
- Premium: None
- Business: 10 tickets
- Enterprise: 25 tickets
Can we buy additional support tickets?
Yes, you can buy additional support tickets on this page.
Can you review test reports?
1. You can submit test reports when asking questions.
2. However, we do not ‘approve’ or ‘confirm’ certificates or other test reports. We are not involved in the testing process; we cannot know on what basis a test report was issued.
Can you review our certificates or other documents?
1. You can submit certificates, declarations and other compliance documents when asking questions.
2. However, we do not ‘approve’ or ‘confirm’ certificates or other documents.
Can you review label files?
1. You can ask questions about product and packaging labelling requirements.
2. You can also request guidance related to label file formats and other technical parameters.
3. However, we do not ‘approve’ or ‘confirm’ label files. That said, you can request a third-party label review from our partners via the platform.
How long does it take to receive a response?
We normally respond within 2 work days (Monday to Friday, excluding public holidays in Hong Kong).
However, we may need 4 to 7 work days to respond to questions that require additional research.
Lab Testing and Other Third-Party Services
Third-party services
1. You can request service quotations from lab testing companies and other third-party service providers via the Compliance Gate Platform.
2. For lab testing, we mainly refer our subscribers to QIMA and TUV Rheinland are accredited* by multiple entities:
*The specific accreditations can vary between branches and labs operated by the companies.
3. These companies can often help you suggest relevant testing requirements and standards for certain products.
4. The terms of service of the service provider apply. We do not provide any warranties or guarantees concerning the provided services. Further, we do not actively monitor the communication between our users and the relevant third parties. You must submit a support ticket if you need our input.
Limitations and risks
You can find a list of limitations and risks related to lab testing and other third-party services in this document.
Why is lab testing necessary?
Lab testing is often necessary to verify if a product complies with a certain regulation, directive, or standard. For example, lab testing can determine if a product complies with certain substance restrictions, or mechanical safety requirements set by product standards. Once the lab test is completed, you will receive a lab test report which indicates if the overall result is pass or fail.
How do we know what tests are required for a certain product?
You must first identify relevant product regulations and other compliance requirements. This can help you understand which safety standards, substance restrictions, and other requirements apply to a particular product. This assessment must be based on the latest version of the relevant legislation, guidance page or other source.
Lab testing companies can also help you assess applicable standards, substance restrictions, and other testing requirements. Another option is to contact market surveillance authorities.
Why do I need a lab test report?
Lab test reports, if the result is passed, serve as evidence that your product is compliant with a certain regulation, directive, or standard. Lab test reports can also be requested by market surveillance authorities or Amazon. In addition, mandatory certificates, declarations, and other documents can only be issued if you have already obtained test reports.
What can be done if the test result is failed?
This indicates that the product does not meet the requirements under relevant regulations, directives, or standards. Here are some examples of why testing can fail:
1. The material contains excessive amounts of restricted substances, such as heavy metals or phthalates.
2. The product failed one or more safety tests.
3. The product fails the flammability requirements.
When this occurs, the course of action depends on what caused the product to fail testing. You may need to redesign the product or change materials.
How does the process work?
1. Request a lab test quotation.
2. The testing company normally responds within a few days with a quotation:
- Suggested tests/standards/substances
- Cost
- Sample quantity
- Lab address
- Booking number
3. If you accept their assessment and quotation, you will be instructed to send a certain number of product samples to a designated address.
4. Samples are sent to the testing company.
5. The final invoice is issued.
6. Lab testing takes place (1 to 3 weeks).
7. A lab test report is issued.
Do you offer testing in-house or through partners?
We do not operate our own testing facilities. Instead, we work with lab testing companies with facilities in Asia, Europe, and the United States.
Which countries/regions are covered by your testing partners?
Our testing partners offer lab testing services for companies selling in the US, EU and UK.
Which testing companies do you work with?
We mainly work with the following two testing companies:
- QIMA
- TÜV Rheinland
We also have a wider network of more specialised labs in the EU, US, UK and Asia.
Where are the testing labs located?
Our lab testing partners have testing facilities in Asia, EU, and the United States. Most of our customers use test lab facilities in the country or region where their products are manufactured.
When should I book lab testing?
You need to verify that your product is fully compliant and safe before you start importing and selling a product. Further, the lab test can either take place before or after mass production, or both.
Pre-production testing: Verify if the product/components/construction is compliant. This can be necessary to avoid an inherently non-compliant product entering production.
Batch testing (post-production): Verify if the final product that is to be placed on the market is compliant.
Note that batch testing is mandatory for certain products.
How do we submit samples to the lab?
The testing company will provide the testing lab address once you have confirmed the quotation.
How many samples do I need to send?
The testing company will confirm the number of samples and/or material quantity required.
How do I know what to test for?
Lab testing companies can assess applicable substance tests and standards based on the following:
- Product type and characteristics
- Materials
- Country/states/market
- Age group
Keep in mind that lab testing companies provide this assessment based on the information they have.
Note: If you intend to ensure compliance with certain US state-level or European national regulations or standards, then this must be clearly stated in your quotation request.
Is the lab testing cost included in the subscription?
No, the Compliance Gate Platform subscription fee does not include lab testing costs. Lab testing fees are charged directly by our lab testing partners.
How much does lab testing cost?
There is no standard rate for lab testing. The cost depends on the following factors:
1. The applicable standards and regulations for the product.
2. The number of unique materials/colors and components that must be tested.
3. Product risk factors (e.g. sharp points, magnets, batteries).
Can you estimate testing costs?
At best, we can share cost examples. However, there are many parameters that impact the lab test cost, which makes it hard to compare the cost of testing one product to another.
What can happen if I don’t have a lab test report?
Without lab test reports you may end up selling a product that is non-compliant and unsafe. Furthermore, market surveillance authorities and Amazon can also request a test report. It is expected that you can demonstrate compliance upon request, or you may face recalls and fines.
What other third-party service providers can we access?
Here are some examples of other third-party services you can access via the platform:
- Label review services
- EU authorised representatives
- Instructions authoring companies
- Translation companies
- Government portals
- Product standards vendors
- SDS services
- Licensing
Monthly Compliance Report / Source Monitoring
What sources do you monitor?
1. We monitor certain legislation texts and other sources in the US, EU, UK, and Canada to keep track of new versions of regulatory requirements covered by the CRL database.
2. You can learn more about monitored sources and our methodology here:
3. We send a report each month summarising our findings and specifying if we deem it necessary to update CRL entries.
4. We also monitor news and announcements concerning new regulations and compliance requirements (not already covered by the CRL database).
Limitations and risks
You can find a list of limitations and risks related to source monitoring in this document.
How often do you review the sources?
We review sources once per month. For example, we review updates from April 2026 in May 2026.
How do you keep the platform up to date?
Please open the US / EU / UK / CA Database documents to learn more about platform updates.
Do you guarantee that the Compliance Gate Platform is always up to date?
No, we cannot guarantee that the Compliance Requirements List database is always up to date as we do not implement changes in real-time. Each month, we review updates from the previous month. We also assess if an update to the affected CRL entry is necessary. As such, CRL entries are only updated after sources have been updated.
It is therefore critical that you always take action based on the latest version of the relevant legislation, guidance documents, and other sources.
Further, document templates are only updated during the annual audit.
How are we made aware of news and updates?
1. We normally send a Monthly Report on the 2nd or 3rd Tuesday of each month.
2. You can also find a list of updates in the Compliance Gate Platform update log.
What is the purpose of the Monthly Report?
The Monthly Report is a compilation of compliance news and updates from monitored sources in the US, EU, UK, and Canada.
It also helps us to decide internally whether we need to update the Compliance Gate Platform.
How does the report benefit us?
The report can help you stay up to date with new and updated compliance requirements, including those that do not result in an update of the Compliance Gate Platform.
How many reports do we receive each year?
You will receive a report each month, for the duration of your subscription.
Data Management & Security
Data Management
The following topics are covered in the Data Management & Security document.
- Security
- How to remove your product data
- Subscription expiry
- Terminate account
- Backups
Security
1. You can find information about security standards, protocols, and measures in the Data Management & Security document.
2. There is always a risk that a website or application is hacked or for other reasons experience data loss. The latter can happen in case of updates or coding errors. We strongly recommend that you always maintain backups of all files generated or uploaded to the Compliance Gate Platform. The Compliance Gate Platform should not be relied upon as a file storage system.
Usage Rights
The Compliance Gate Platform can either be used for your own organization, or on behalf of the customers of your organisation.
Your organisation (Standard)
1. This means that you can only use the Compliance Gate Platform to create requirements lists, documents, label files, and request support for your own company.
2. This is suitable for most brands, manufacturers, importers, and e-commerce companies using the platform for their own products.
Third-parties (Expanded)
1. You can use the Compliance Gate Platform to create requirements lists, documents, label files, and request support on behalf of your customers:
2. You can use the Compliance Gate Platform to support paid consulting work or support requests from your customers. Examples include:
- Freight forwarders that need to identify requirements for their customers
- Consultants that need to create requirements lists, documents, or label files as part of a project
3. However, the following is prohibited:
- Selling individual requirements lists
- Selling individual document templates
- Selling account access to individual users
4. It is important that your customers read the Feature Overview, US / EU / UK / CA Databases, and the FAQs to understand the scope and limitations of the Compliance Gate Platform.
Payments & Subscription
Can I pay with my Visa or MasterCard?
Yes, we offer credit card payments through PayPal or Stripe. Customers based in certain countries may be required to create a PayPal account in order to make a Credit Card transaction. This is free and only takes a moment. You just need to add your email address and agree with the PayPal Terms of Service. With Stripe, you don’t need to create an account.
Can I pay with PayPal?
Yes, you can pay with your balance, bank account, or credit card through PayPal.
Do you offer a safe payment method?
Yes, all payments on this website are processed by PayPal or Stripe, which use an SSL certificate to encrypt your data.
Will my subscription get automatically renewed?
If you subscribed on or after 2025-05-08, your card will be billed automatically on the date of expiration. However, you can cancel your subscription at any time and, in this case, your card will not be billed again.
Do you have a refund policy?
The subscriber has the right to cancel the purchase up to, but no later than, 14 days from the order date. However, this only applies if the subscriber has not yet logged into the platform and/or used the platform to create compliance requirements lists or templates.
Can I get a partial refund if I cancel my subscription before the expiration date?
No, we don’t offer partial refunds.
Will I receive an invoice for my company?
Yes, it’s delivered together with the payment confirmation email.
Can I pay via bank transfer?
Yes, you can contact us to request an invoice if you prefer to pay via bank transfer. In this case, we will only activate the account once we have received the payment.
Can the subscription activation be delayed?
Yes, you can request a delay in activation after subscribing (if done within 7 days).
1. Reply to the order confirmation email that you will receive after signing up (within 7 days). We will then put your subscription on hold until you request activation.
2. You can set an activation date now or contact us again when you are ready to start using it.
3. You can delay the activation for up to 24 months after your order.
Can the subscription be paused?
No, you cannot pause a subscription that is in use or was not delayed for activation within 7 days after your order.
Terms of Service and Privacy Policy
Where can I find your Terms of Service?
Follow this link to read our Terms of Service.
Where can I find your Privacy Policy?
Follow this link to read our Privacy Policy.