Food contact materials and articles imported or manufactured in the European Union are subject to various regulations. These regulations, wihch are often collectively referred to as Food Contact Materials (FCM) regulations, cover substance restrictions, documntation, labelling, testing and other requirements.
In this guide, we list many of the key EU FCM regulations and explain when these are relevant, and break down many of their respective requirements.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
EU Food Contact Materials Framework Regulation (EC) 1935/2004
Food contact materials (FCM) refer to any materials and articles intended to come into contact with food. Regulation (EC) 1935/2004 sets safety principles for FCM manufactured, imported, and sold in the EU.
Covered products should not:
a. Release their constituents into food at levels harmful to human health
b. Change food composition, taste, and odour
c. Bring about changes in the taste or odour of the food
Product scope
This regulation covers food contact materials and articles that:
a. Are meant to come into contact with food
b. Are already in contact with food
c. Are expected to come into contact with food under normal usage conditions
The regulation does not cover:
a. Antique articles and materials
b. Covering or coating materials as food constituents
c. Fixed public or private water supply equipment
Requirements
The regulation provides a general framework for FCM so that they do not harm human health or otherwise alter the food’s characteristics. It generally requires:
- Adherence to good manufacturing practice (GMP)
- Declaration of Compliance
- Traceability information
- Compliance with substance migration limits via testing
Annex I of the regulation lists groups of articles and materials that may be covered by the specific measures laid out in Article 5. This guide lists the specific measures adopted under this regulation that we could find.
Labelling requirements
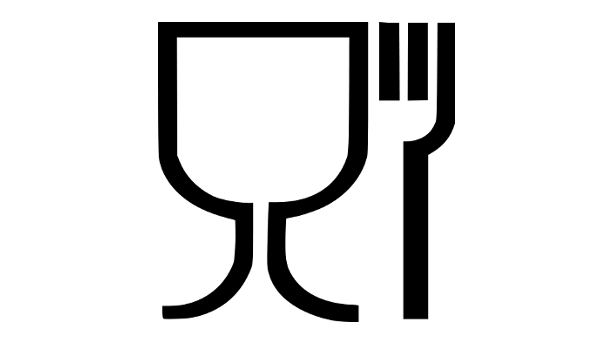
Article 15 of the regulation covers labelling requirements for FCM. Specifically, it requires companies to label their products with the following information:
a. Traceability information:
- Company name or trade name
- Company address or registered office
- Adequate identification information to ensure traceability as described in Article 17
b. Information regarding FCM not yet in contact with food when sold:
- The words “for food contact”, or
- A specific indication of the material’s use, or
- The FCM symbol as provided in Annex II
Note that the above information is only necessary for articles for which it is not obvious that they come into contact with food (e.g. it is normally not necessary for a fork or a knife).
c. Special instructions for safe and proper use, if necessary.
d. Further information (e.g. name and quantity of released substances) may be required for active FCM.

Plastic FCM Regulation (EU) 10/2011
This regulation is a specific measure of the EU Food Contact Materials Framework Regulation. It sets manufacturing, substance restrictions, and labelling requirements regarding plastic materials and articles that:
- Are meant to contact food
- Already contact food
- Can be expected to contact food
Product scope
This regulation applies to the following products:
a. Plastic materials and articles
b. Multi-layer plastic articles and materials held together by adhesives
c. Printed or coated plastic materials and articles
d. Printed or coated multi-layer plastic materials and articles with adhesives
e. Plastic layers in multi-material, multi-layer articles
The regulation does not cover:
- Ion exchange resins
- Rubber
- Silicones
Substance restrictions
You should comply with the substance restrictions in this regulation by:
a. Only using the authorised substances in Annex I to manufacture plastic materials and products
b. Using pure, high-quality substances in plastics to ensure safe, intended use – even from recycled sources
c. Adhering to specific substance requirements set out in Articles 9 to 14 of the regulation
Declaration of Compliance
The regulation requires the business operator to issue a Declaration of Compliance for:
- Plastic materials and articles
- Products from intermediate stages of manufacturing
- Substances meant for use in manufacturing those products
The business operator should ensure the Declaration of Compliance contains the information listed in Annex IV.
Labelling requirements
Per Article 4, you should comply with the labelling requirements in the EU Food Contact Materials Framework Regulation.
Additionally, according to Article 14a, if you sell a final food contact article that is made of plastic and meant for repeated use, you should provide users with:
a. Suitable instructions for slowing down the article’s deterioration
b. Description of visible changes
c. A warning indicating that the migration of substances through damage or misuse may render the article unsafe for further contact with food
Recycled Plastic FCM Regulation (EU) 2022/1616
This regulation sets requirements concerning the development, usage, and placement in the market of recycled plastic FCM.
Product scope
This regulation covers the following types of products:
a. Plastic FCM that contains, or is manufactured from plastic originating from waste
b. Plastic recycling technologies for use in plastic FCM
c. Plastic FCM intended for recycling
Requirements
The regulation sets the following types of requirements:
a. Recycling requirements
b. Documentation (e.g. Declaration of Compliance), instructions, and labelling
c. Collection and pre-processing
d. Decontamination of recycled plastic
e. Post-processing and use of recycled plastic articles and materials
f. Operation of recycling schemes
g. Development of a novel technology for processing recycled plastic
Declaration of Compliance
Annex III contains templates for two different Declarations of Compliance:
a. PART A – Declaration of Compliance to be used by recyclers
b. PART B – Declaration of Compliance to be used by converters if the converted plastic material contains recycled plastic
Use of Bisphenol A in FCM Regulation (EU) 2024/3190
This regulation restricts the use of Bisphenol A, its salts, and other dangerous bisphenols and their derivatives.
Product scope
This regulation applies to the following groups of food contact materials and articles:
- Adhesives
- Rubbers
- Ion-exchange resins
- Plastics
- Printing inks
- Silicones
- Varnishes and coatings
Substance restrictions
The regulation prohibits:
a. BPA and its salts in the manufacture of food contact materials (FCM) and articles
b. Residual BPA in FCM and articles manufactured using another bisphenol or bisphenol derivative
c. The usage of other hazardous bisphenols other than BPA or its derivatives
Note, however, that BPA may be used as a monomer or starting substance in liquid epoxy resins, as long as its migration into food remains at undetectable levels.
Declaration of Compliance
The regulation requires business operators to provide a declaration of compliance with:
a. Their food contact materials and articles not yet in contact with food, and
b. Bisphenols intended for use as starting substances or monomers
The business operators should ensure the Declaration of Compliance contains the information listed in Annex III.
Authorisation and reporting requirements
The regulation contains:
a. Authorisation requirements for using hazardous bisphenols other than BPA or other derivatives
b. Reporting requirements regarding the use of alternative but hazardous substances other than BPA or their derivatives
Ceramics Directive 84/500/EEC
This directive sets requirements for ceramic articles meant to come into contact with food.
Product scope
The directive covers ceramic articles that may be:
- Glazed
- Enamelled
- Decorated
Substance restrictions
Article 2 of the directive specifies the substance restrictions for the migration of lead and cadmium from ceramic articles, as summarised in the table.
| Category | Ceramic articles | Lead | Cadmium |
| Category 1 | Articles with an internal depth not exceeding 25 mm, including:
a. Fillable articles b. Non-fillable articles |
0.8 mg/dm2 | 0.07 mg/dm2 |
| Category 2 | a. All other fillable articles | 4.0 mg/litre | 0.3 mg/litre |
| Category 3 | a. Cookware
b. Packaging and storage vessels with a capacity of more than 3 litres |
1.5 mg/litre | 0.1 mg/litre |
Declaration of Compliance
The directive requires sellers or manufacturers of ceramic articles to issue a Declaration of Compliance. You can find more information in Annex III of the directive.
Regenerated Cellulose Film Directive 2007/42/EC
This directive sets forth requirements for materials and articles made of regenerated cellulose film intended to come into contact with food.
In particular, it requires that only authorised substances be allowed for use in manufacturing regenerated cellulose films. Additionally, the printed surfaces of regenerated cellulose film should not come into contact with foodstuffs.
Product scope
This directive covers regenerated cellulose film meant to come into contact with foodstuffs and either:
a. Is a finished product
b. Forms part of a finished product that contains other materials
Substance restrictions
The directive provides two lists of substances authorised for use in manufacturing regenerated cellulose film. The first concerns uncoated regenerated cellulose film, and the second covers the coated variety. You can find the details in Annex II.
Labelling requirements
Importers and manufacturers should label their FCM made of regenerated cellulose film accordingly when special usage conditions are necessary.
Written Declaration
Importers and manufacturers need to draft a written declaration for regenerated cellulose film intended for food contact, except when the product is, by its nature, clearly intended for this use. You can find information about the written declaration in Article 16(1) of Regulation (EC) No 1935/2004.
Active and Intelligent FCM Regulation (EC) 450/2009
This regulation sets specific requirements for active and intelligent FCMs meant to come into contact with food.
It mandates that importers and manufacturers ensure that active and intelligent FCMs are acceptable and functional for use. It also sets substance restrictions, labelling and documentation requirements
Product scope
This regulation covers active and intelligent FCM.
Substance restrictions
The regulation restricts the migration of substances from components to food to a maximum of 0.01 mg/kg.
It also claims that you may only use substances on the ‘Community list’ in components of active and intelligent FCM. However, we were unable to find any such ‘Community list’ through our research.
Labelling requirements
Importers and manufacturers should label active and intelligent non-edible FCM and their parts with the following:
a. The words “DO NOT EAT”; and
b. The symbol in Annex I, always and where technically feasible
The above information must be clear, permanent, and printed in a font size of at least 3 mm.
Declaration of Compliance
Annex II of this regulation lists the information that importers and manufacturers must provide in their Declaration of Compliance.
Polyamide and Melamine Plastic Kitchenware from China or Hong Kong SAR (China) Regulation (EU) 284/2011
This regulation establishes conditions and methods regarding the importation of the following products made in or delivered from China or Hong Kong SAR (China):
- Polyamide kitchenware
- Melamine plastic kitchenware
It mandates that importers and manufacturers comply with substance restrictions, as well as notification and declaration requirements.
Product scope
This regulation covers polyamide and melamine plastic kitchenware that originates in or has been consigned from China or Hong Kong SAR (China).
Substance restrictions
Here are the substance restrictions for polyamide and melamine plastic kitchenware as set by the regulation:
a. Polyamide kitchenware – the sum of primary aromatic amines should not exceed a detection limit of 0.01 mg/kg of food or its simulants
b. Melamine plastic kitchenware – the quantity of formaldehyde released into food or its simulants should not exceed 15 mg/kg of food
Notification requirements
The regulation requires importers of covered products to notify the relevant authorities two days before their products from China or Hong Kong SAR (China) arrive in the EU.
Declaration
Businesses can import polyamide and melamine plastic kitchenware into the EU only if they provide the relevant authorities with a completed declaration confirming the products’ compliance with relevant substance restrictions.
The Annex provides a template for this declaration, which should also include a lab test report.
Epoxy Derivatives Restriction Regulation (EC) 1895/2005
This regulation restricts and prohibits the use of certain epoxy derivatives. It also mandates importers and manufacturers to provide a written declaration.
Product scope
This regulation covers FCMs that contain, or are manufactured with, one or more of the following substances:
- ‘BADGE’ and some of its derivatives
- ‘BFDGE’
- ‘NOGE’
Substance restrictions
The regulation restricts the migration limit for the usage of BADGE in the manufacture of FCM.
It lists the following specific migration limits for BADGE and its derivatives in Annex I:
a. The total migration limit of the following substances:
- BADGE [= 2,2-bis(4-hydroxyphenyl)propane bis(2,3-epoxypropyl) ether]
- BADGE.H2O
- BADGE.2H2O
should not exceed 9 mg/kg in food or its simulants or 9 mg/6 dm2 in the cases specified by Article 7 of Commission Directive 2002/72/EC.
b. The total migration limit of the following substances:
- BADGE.HCl
- BADGE.2HCl
- BADGE.H2O.HCl
should not exceed 1 mg/kg in food or its simulants or 1 mg/6 dm2 in the cases specified by Article 7 of Commission Directive 2002/72/EC.
The regulation also prohibits the usage and/or presence of BFDGE and NOGE in the manufacture of food contact materials and articles.
Written Declaration
Importers and manufacturers should provide a written declaration with their FCM that contains BADGE and its derivatives. You can find more information in Article 5 of the regulation.
Teats and Soothers Directive (93/11/EEC)
This directive sets limits and test methods for the release of N-nitrosamines and N-nitrosatable substances from rubber or elastomer teats and soothers.
It is featured in this guide because it is listed as “legislation on specific substances” in the guidance page concerning Food Contact Materials legislation published in the EU website.
Product scope
This directive covers the following products:
- Elastomer or rubber teats
- Elastomer or rubber soothers
Substance restrictions
Per Article 2 of the directive, teats and soothers should not transfer N-nitrosamine and N-nitrosatable substances in the following amounts to the release-test liquid specified in Annex I:
a. The total release of N-nitrosamines should not exceed 0.01 mg/kg of the parts of the elastomer, rubber teats, or soothers
b. The total release of N-nitrosatable substances should not exceed 0.1 mg/kg of the parts of the elastomer or rubber teats or soothers
Good Manufacturing Practice for FCM Regulation (EC) 2023/2006
This regulation requires manufacturers to implement a sound and approved system during the manufacturing process of products.
While the regulation applies to the manufacture, processing, and distribution of FCM, it does not apply to manufacturing starting substances.
Product scope
The regulation covers articles and FCM listed in Annex I of Regulation 1935/2004, such as the following:
- Adhesives
- Ceramics
- Glass
- Plastics
- Regenerated cellulose
Requirements
According to Article 4 of the regulation, manufacturers should carry out their operations per the general requirements regarding:
- Quality assurance
- Quality control
- Documentation
Manufacturers should also adhere to the detailed rules in the Annex on good manufacturing practice regarding:
a. Applying printing inks to the non-food-contact side of FCMs or articles
b. Requirements for quality assurance systems at recycling facilities that manufacture recycled plastic per the Recycled Plastic FCM Regulation
c. The reprocessing of plastics covered by the Plastic Materials Regulation
Substance restrictions
Per Part A of the Annex, manufacturers should ensure that:
a. The substances in the printing inks applied to the non-food contact side of the FCM do not migrate to the food-contact side
b. The concentration of printed inks does not result in a violation of the requirements set out in Article 3 of the EU FCM Framework Regulation. That is, the printed inks should not:
- Harm human health
- Modify the food composition in an unacceptable way, or
- Negatively affect the taste, smell, or appearance of the food
Guidance Documents and Resolutions
Guidance and other relevant documents may be helpful when it comes to applying the requirements of the regulations (e.g. ensuring that a product does not contain restricted substances above the allowed limits).
For example, in our experience, in certain cases, lab testing is carried out based on resolutions and guidance documents. This can happen especially when regulations are more open-ended, or when a standard or substance restriction does not exist for a specific food contact material.
Multi-language versions of brochures and guidance documents
On this page, the European Commission lists several brochures and guidance documents, providing versions in multiple languages such as English, German, French, and more. Here are the titles of the documents in English:
a. Brochure: “Food Contact Materials”
b. Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food
c. Declaration to be provided for every consignment of polyamide and melamine plastic kitchenware originating in or consigned from People’s Republic of China and Hong Kong Special Administrative Region, China
d. Union Guidance on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food as regards information in the supply chain
Food contact materials technical guidance documents
The European Union Reference Laboratory for Food Contact Materials (EURL-FCM), which aims to provide assistance to the EU and its member states, lists several FCM technical guidance documents on this page.
Here is a list of the documents that you can find on the above-mentioned page:
a. Testing conditions for kitchenware articles in contact with foodstuffs: Plastics, Metals, Silicone and Rubber, Paper & Board
b. Roundtable Workshop on the Determination of MOAH in Infant Formula (Summary and Minutes)
c. Guidance on sampling, analysis and data reporting for the monitoring of mineral oil hydrocarbons in food and food contact materials
d. Guidance on characterisation of the composition of plastic multi-layers
e. Task force on migration modelling (Guidelines, List of substances)
f. Guideline on testing migration of primary aromatic amines from polyamide utensils and for formaldehyde from melamine-based kitchenware in support of Regulation (EU) No 284/2011 on imports from China and Hong-Kong
g. Calculator for the correction of the experimental specific migration for comparison with the legislative limit
h. Guidelines on test conditions for kitchenware
i. Guidelines to evaluate method performance and conduct validation studies of analytical methods for FCM
Resolution ResAP(2004)5 on silicones used for food contact applications
The Committee of Ministers from the Council of Europe put forth principles in “Resolution ResAP(2004)5 on silicones used for food contact applications”. This includes:
a. Using a certified Quality Assurance System (e.g. ISO 9002)
b. Conduct migration testing according to specific directives (e.g. Directive 97/48/EEC)
c. Provide appropriate labelling
The Committee recommends that EU member states take these principles into account in their laws and regulations regarding the usage of silicones in food contact applications
National European FCM Regulations
Several European countries implement domestic FCM regulations. Below, this section provides some examples.
LFGB (Germany)
The LFGB is the German abbreviation for Germany’s Foodstuff and Feedstuff Code, which is considered the most important food safety management act in the nation. It ensures the safety of food and food-related products in the country.
Foodstuffs and food-related products are required to pass the corresponding tests and be compliant with the LFGB to be sold in Germany.
Generally speaking, the LFGB is considered to be stricter than other EU FCM regulations. It regulates a wide range of substances and materials, such as the following:
- Polymers
- Silicon
- Paper
You can find a comprehensive list of LFGB-restricted substances on this page.
DGCCRF (France)
DGCCRF is the French abbreviation for France’s Directorate General for Competition Policy, Consumer Affairs and Fraud Control, which regulates and controls products within the French market at many levels, including production, import, and distribution. One of DGCCRF’s main focuses is regulating foodstuffs and food-related products.
The DGCCRF sets requirements for FCM made from materials such as the following:
- Metal
- Ceramic
- Plastic
- Paper
- Wood
- Glass
- Organic materials made from synthetic fibres
- Organic materials made from plant fibres
It restricts the usage of the following substances in most articles:
- Aluminium
- Cobalt
- Arsenic
FCM lab testing
Importers and manufacturers should have their products tested and receive a test report to prove compliance with FCM regulations requirements (e.g. substance restrictions and migration limits).
Also, different regulations and EN standards may apply to different materials and products, such as paper and board, plastics, or active and intelligent materials.
Test methods
Here are some examples of test methods relevant to FCM:
a. Overall migration tests (e.g. EN 1186-1)
b. Lead and cadmium migration tests for ceramic FCM (Directive 84/500/EEC)
c. Plastic constituent and colouring migration tests for regenerated cellulose film (Directive 2007/42/EC)
Lab testing companies
This subsection lists some companies that offer FCM testing against EU regulations and relevant testing methods:
Compliance Risks
Food contact products and materials manufactured outside the EU are not exclusively made to comply with European Union FCM regulations. Here are some compliance risk scenarios:
a. Plastic lunch boxes containing excessive amounts of BPA
b. Wooden coatings or paints containing phthalates
c. Bamboo melamine composites contain excessive amounts of toxic chemicals
d. Stainless steel drink bottles containing excessive amounts of lead and cadmium
Importing non-compliant food contact materials can result in fines and a forced recall. It’s therefore essential to only work with suppliers having a certain degree of ‘substance control’ – meaning that they can procure or produce compliant materials. This cannot be taken for granted when sourcing products from manufacturers outside the EU.
The only way to assess a supplier’s capability to produce compliant FCM products is by assessing their existing compliance track record. In other words, do they have some existing EU FCM test reports proving that they have made compliant products in the past? If not, then they are less likely to have the capability to manufacture a compliant product.
That said, third-party lab testing is ultimately required when importing and selling food contact products in the EU. You cannot use existing test reports provided by the supplier to prove that your imported product is compliant.






















.png)
.png)
.png)




Hello, can I contact you by email? I see an offer for a free trial consultation, but unfortunately my level of English does not allow me to speak well. I have studied your presentations and would like to ask some questions. Thank you.
Hello. Are ice packs considered to be FCM?
Will the ice cubes be consumed? If so, then quite likely.
Hi..
What test parameters are required for silicon mold? are there any other requirements?
Will reusable polyethylene plastic straws need to be subjected to sensory analysis, if already testing for overall migration, specific migration or heavy metals and of primary aromatic amines?
for sale in the EU, by the way
HI
What sanitary authorisation paperwork do i need to produce to send industrial bakeware to Italy
The bakeware is made from Aluminium. Stainless Steel or Aluminised Steel
Do these regulations apply to pet products? (Imagine a plastic food bowl for dogs)
Hi there, I intend to ship kitchenware products to Amazon UK. Just wondering is LFGB mandatory and whether you provide testing service.
Hi Tasha,
LFGB is from Germany. That said, there are FCM regulations in the UK as well.
Hello,we’re a trading company and going to ship a container with Lemon Squeezers and Papper Mills from China to Germany via Rotterdam Port.
Both are manual,non-electric,HS:82100000
Since those are FCM(food contact materials),is any test certificate be required by Dutch or German Customs?E.g. EC 1935/2004 or LFGB etc.Thank you.
What are legal compliance requirements for PET granules ( Food Contact Materials) manufactured in Belgium Plant.
Dear. Fredrik Gronkvist
Thank you for your useful informations
I’m working at testing institute (almost same as SGS and BV)
I’d like to try setting up the testing FCM
Can I contact with you by e mail?
Thank you
Hello Gy Ryu,
Thanks, I have replied your email
Hi.
Do wine pumps and aerators fall under Food Contact requirements?
If these are in contact with the wine then I would guess so
Hello you think the bootle with LFGB test reports it can send to france?
Hi Abby,
As far as I know, LFGB is a German regulation/standard
Hi Abby,
every country has own migration limits and method to test. So depends on the customer who buying it. In my opinion if you can eat or drink from a product in Germany than it also need to be possible in France. The law will change soon so it will be more harmonised.
Hi,
I try to get my item to be tested and acquire DOC (LFGB).
The country to export is Germany and the item is water bottle (no water inside).
Do you provide testing services as well?
Many thanks
Junghun
Hi Jonghun,
We can introduce you to testing companies